Adverse Event reporting information can be found in footer
Request a Meeting

PENTASA formulations are licensed for achieving and maintaining remission in children from the age of 6 and upwards:2-5
Paediatric FormulationsPrimary endpoint was mean PUCAI score between the two arms (OD and BD dosing) at week 6. At week 6, there was no difference in the median PUCAI score between the OD and BD groups (p=0.48).*
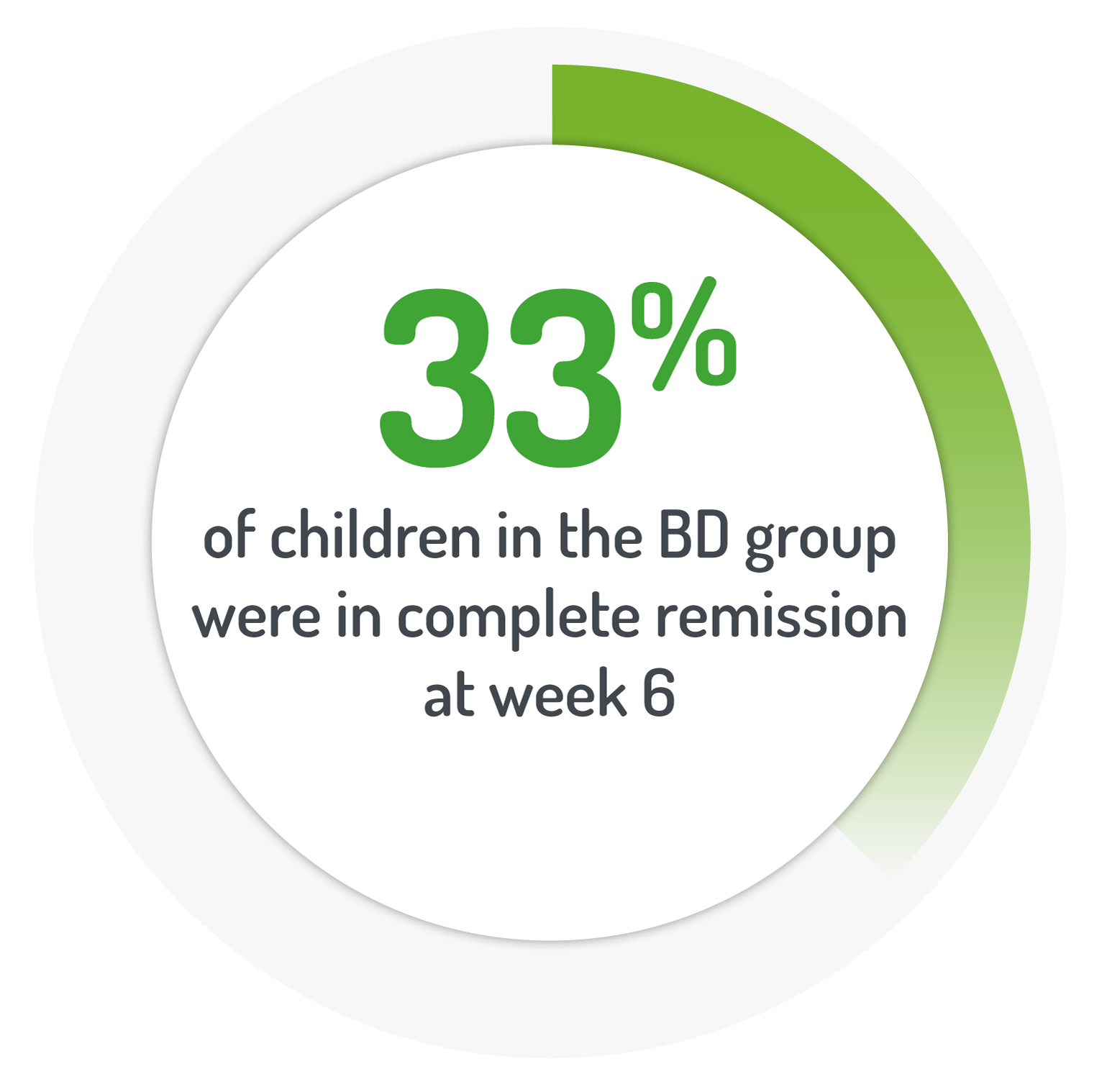
PUCAI<10 points and a change of at least 10 points from baseline
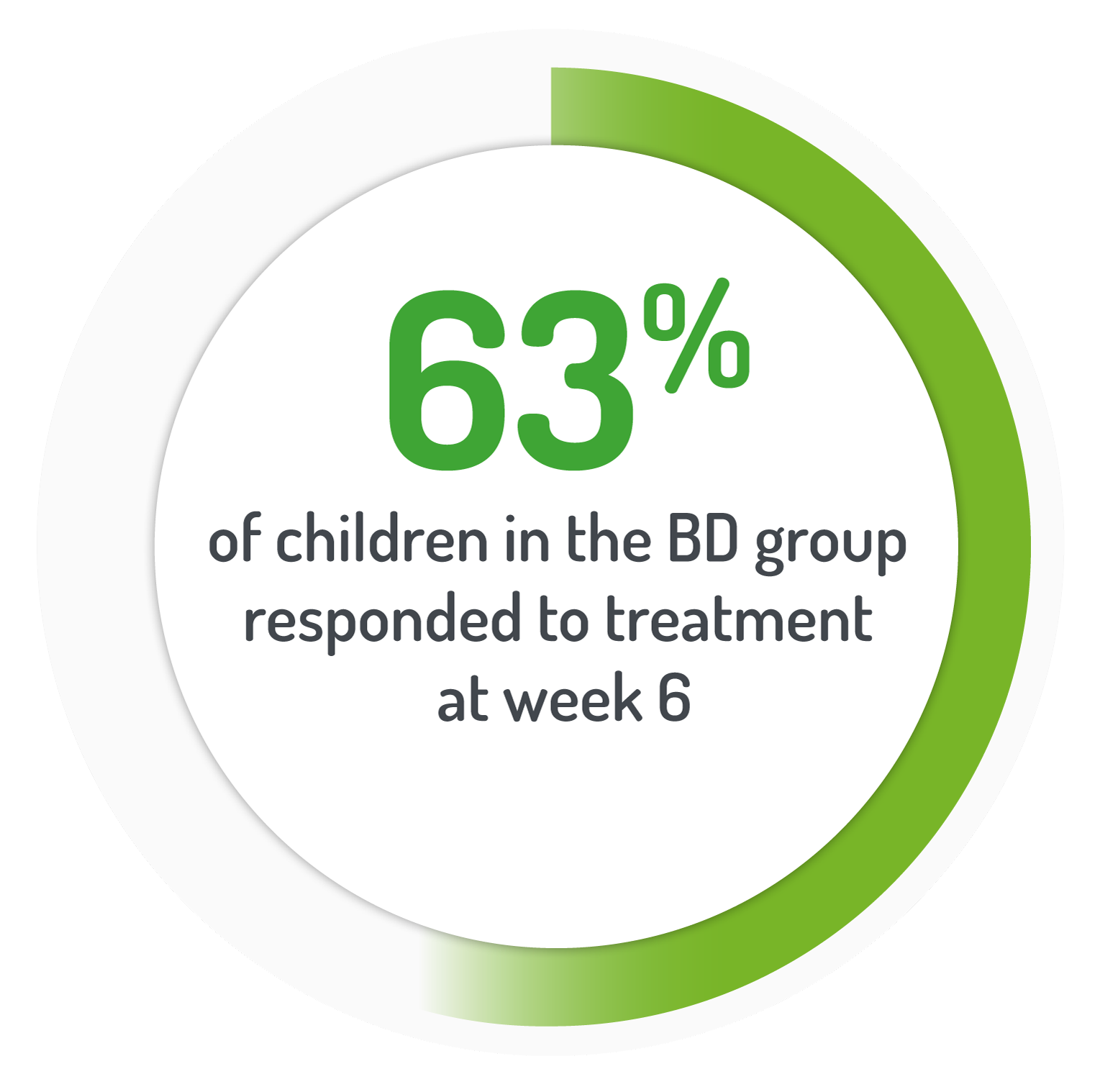
PUCAI≥10 points (active disease) but an improvement of at least 20 points
*PENTASA® is licensed to be taken in divided doses only in paediatrics. †Weight-based dosing based on 75mg/kg/day.
PUCAI = Paediatric UC disease activity index, UC = Ulcerative colitis.
Note: PENTASA® OD is not licensed for paediatric use in the UK.
1g PENTASA® sachet† BD for 6 weeks
Study Summary
| Patient Numbers | Dose amount | Dose frequency | Rectal dose |
|---|---|---|---|
| 40 | 15-<20kg | 1,000 mg (500 mg, 500 mg) | Twice-daily |
| 20-<30kg | 1,500 mg (1,000 mg, 500 mg) | ||
| 30-<40kg | 2,000 mg (1,000 mg, 1,000 mg) | ||
| ≥40kg | 3,000 mg (1,500 mg, 1,500 mg) |
ITT = Intention to treat.
Key Inclusion Criteria
Key Exclusion Criteria
Safety results:
*PENTASA® (500 mg tablets, 1g, 2g and 4g sachets) is licensed for children from the age of 6 and older, and given in divided doses.
SAEs = Serious adverse events
PENTASA formulations are licensed for achieving and maintaining remission in children from the age of 6 and upwards:
Job Code: UK-PA-2000023 - Date of preparation: October 2020