


Adverse Event reporting information can be found in footer
Request a Meeting
The statements in this quality standard apply to children and young people aged 5 to 18 years. Children are generally expected to be dry by a developmental age of 5 years. It is important that children aged 5 to 7 years are not excluded from the management of bedwetting on the basis of age alone, and therefore this quality standard addresses the needs of these younger children alongside older children and young people1.
Children and young people with bedwetting that hasn’t improved after changing their daily routine (and their parents or carers if appropriate) discuss possible treatment (such as a bedwetting alarm or medication) with their healthcare professional.
The choice of initial treatment should be informed by the comprehensive initial assessment, and should take into account the preference of the child or young person and, if appropriate, their parents or carers. Factors such as age, associated functional difficulties and disabilities, financial burdens and living situations may affect their preferences1.
Children and young people whose bedwetting has not responded to courses of initial treatments are referred for a specialist review1.
NICE have developed a guideline for desmopressin treatment for bedwetting in children and young people:2
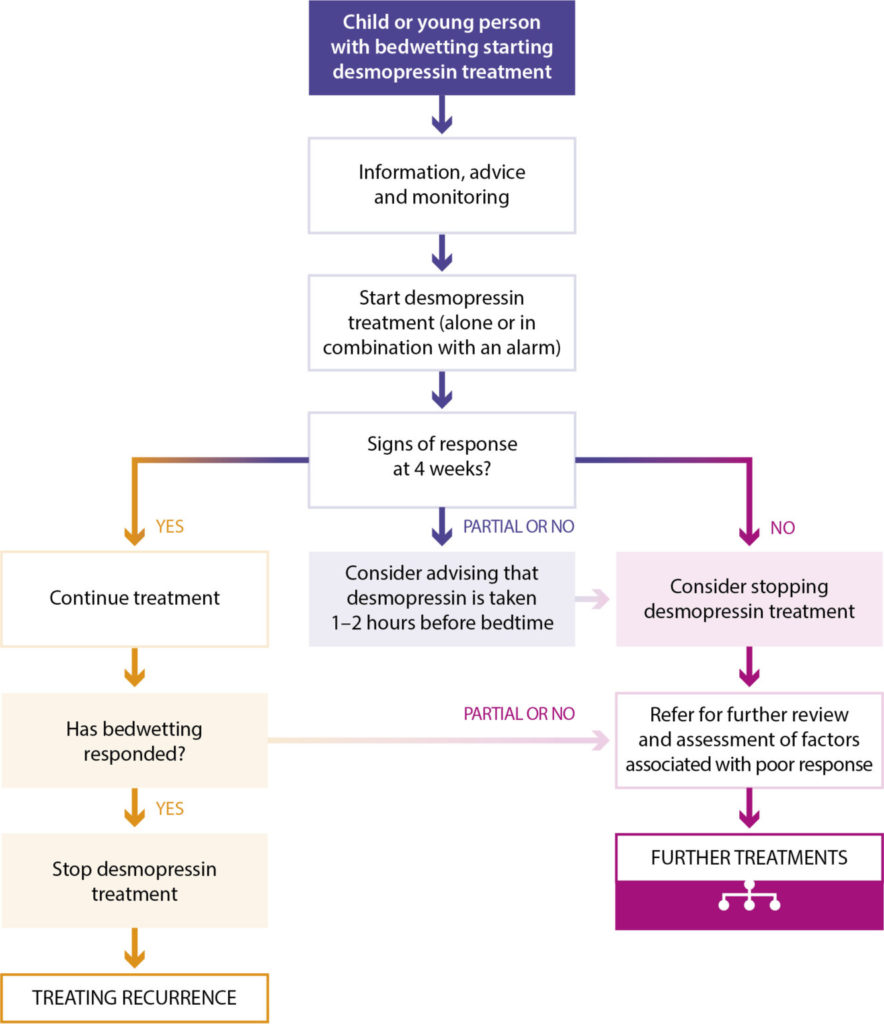
Adapted from NICE 2017. Desmopressin treatment for bedwetting in children and young people.
(desmopressin [as acetate] oral lyophilisate)
Studies have compared desmopressin treatment with alarm therapy in children. Based on patients who completed a 6-month treatment, success was achieved in 76.8% and 61.8% of children in the desmopressin and alarm groups, respectively5. At 12 months, 77.8% of desmopressin patients and 75% of alarm patients had success. Long-term success rate was significantly higher with desmopressin melt vs. alarm (68.8% vs. 46.2% [p=0.006]; intention to treat analysis)5.
While it has been shown that desmopressin and use of an alarm show comparable efficacy in the treatment of primary nocturnal enuresis, drop-out rates from children using the alarm group can be high, indicating the importance of considering family motivation before selecting treatment, to ensure optimal outcome6.
The NICE Approach provides a summary of the NICE clinical guidelines for the assessment and management of NE.
Download the summary here to find out more.
Job Code: UK-MN-2200029 - Date of preparation: March 2023