


Adverse Event reporting information can be found in footer
Request a Meeting
DesmoMelt is well accepted by children of various ages and facilitates early intervention2, providing an effective and convenient solution to PNE and helping children and families return to a normal life.
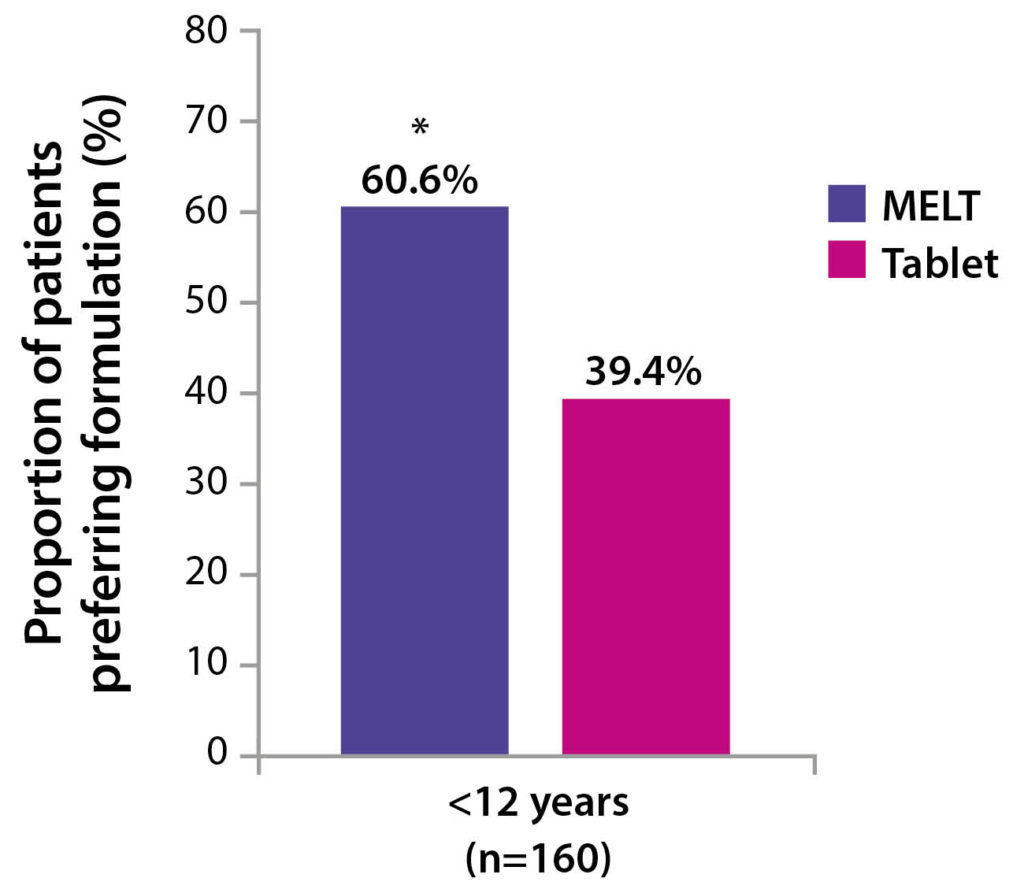
A statistically significant preference for DesmoMelt has been shown in children aged 5-11 years2. (For patients
<12 years, 60.6% patients preferred the melt formulation [p=0.009])2
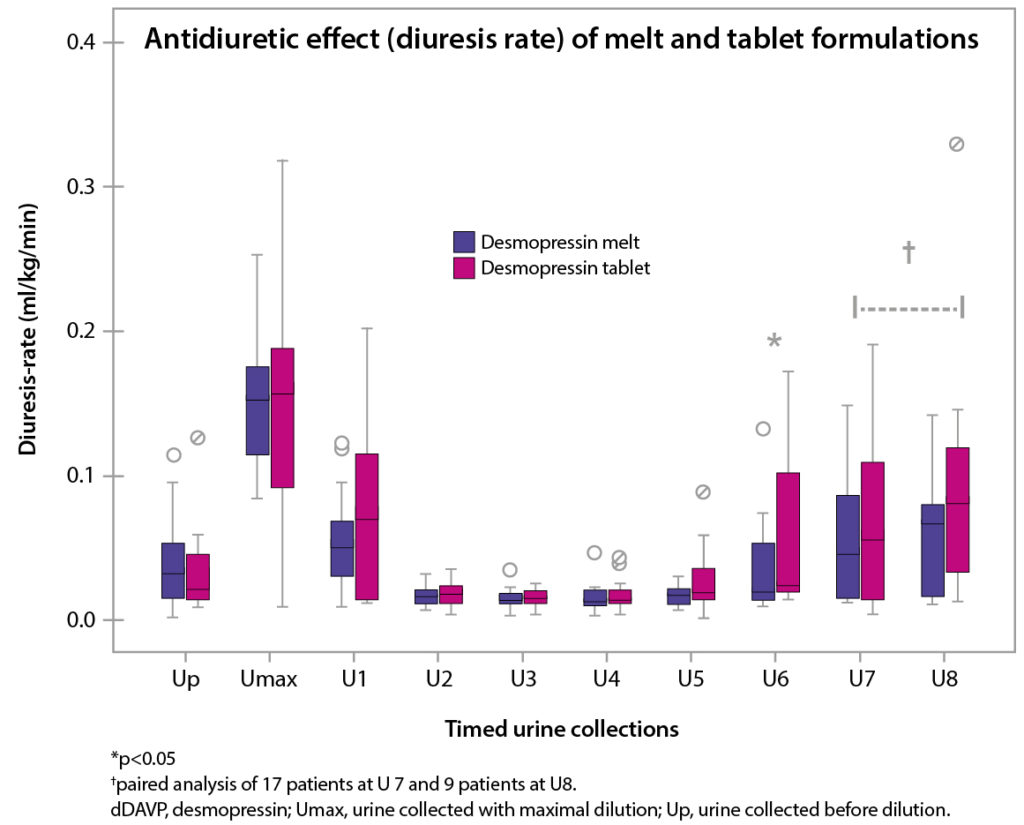
Compared with tablets, DesmoMelt provides a significantly superior antidiuretic effect and concentrating capacity 3-8 hours after dosing3.
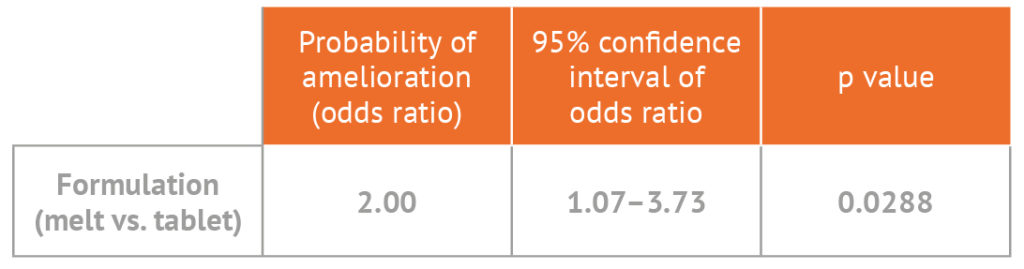
The probability of a reduction in bedwetting episodes is twice as likely with DesmoMelt4
Tablet consumption may require 60 mL of water (approximately a quarter of the expected bladder capacity of a 7 year old) which may also impact the efficacy of desmopressin4.
A single dose (120mcg) of DesmoMelt should be taken before bedtime for a period of 3 months, followed by a one-week drug-free period to assess results. If symptoms persist, the treatment cycle should be repeated1.
For more information about the benefits of DesmoMelt, download our short leaflet.
Side-effects include; headache (common (≥ 1/100 to < 1/10), stomach pain and nausea (≥ 1/1,000 to < 1/100). Isolated cases of allergic skin reactions and more severe general allergic reactions have been reported. Very rare cases of emotional disorders including aggression in children have been reported. Treatment with desmopressin without concomitant reduction of fluid intake may lead to water retention/hyponatraemia with accompanying symptoms of headache, nausea, vomiting, weight gain, decreased serum sodium and in serious cases, convulsions.
For further information on the safety profile of DesmoMelt please refer to the full SmPC DesmoMelt 120mcg oral lyophilisate – Summary of Product Characteristics (SmPC) – (emc) (medicines.org.uk)
Job Code: UK-MN-2200029 - Date of preparation: June 2024