
Control at Your Fingertips

Adverse Event reporting information can be found in footer
Request a Meeting
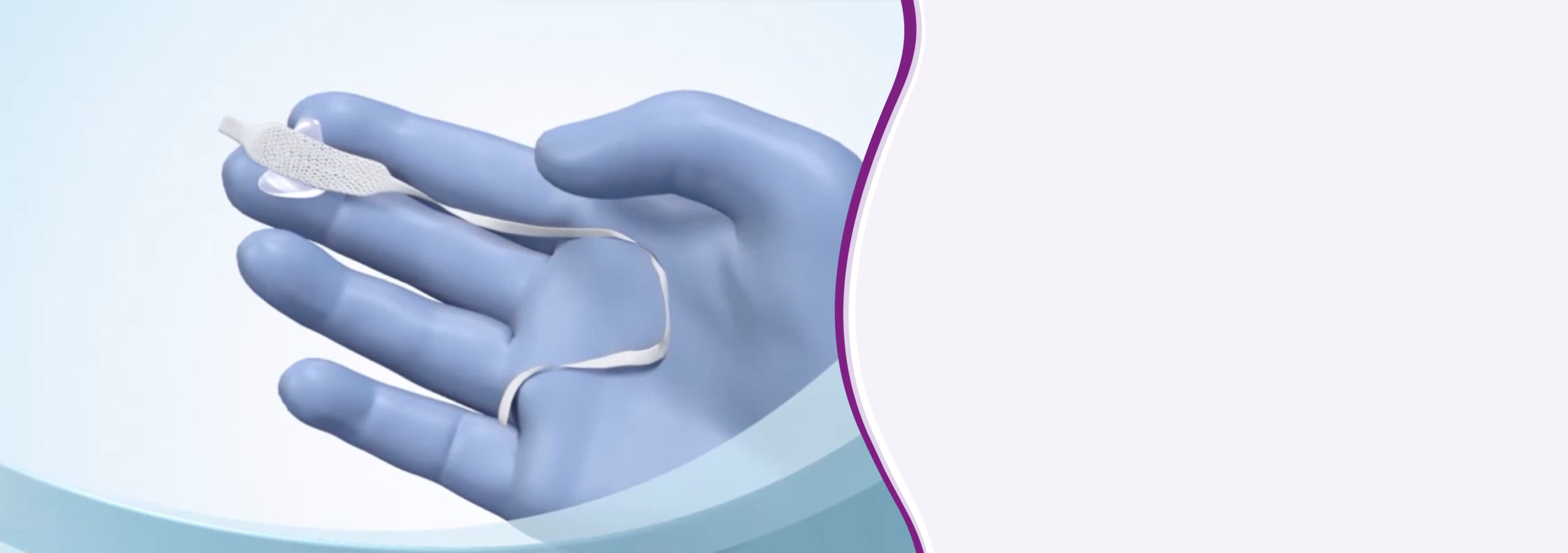
Propess is a single application, removable, controlled-release vaginal delivery system containing 10mg dinoprostone which is a prostaglandin E2. It is indicated for the initiation of cervical ripening in women from 38 weeks gestation as part of the induction of labour process.1
When Propess is inserted into the vagina, dinoprostone is released at a mean rate of approximately 0.3 mg/hour and drug release continues as long as the product is in situ in the vagina (for a period of up to 24 hours). The system is equipped with a withdrawal tape that facilitates convenient and easy removal when active labour begins or removal of the system is indicated.1
Job Code: UK-RMMH-2000023 - Date of preparation: March 2022