Glypressin (terlipressin acetate) statement
Ferring will be removing the brand name GLYPRESSIN® (terlipressin acetate) from our 1 mg powder and solvent for solution for injection vials, and our 0.12 mg/ml solution for injection ampoules. The product will be sold under its generic name, terlipressin acetate, from May 2024.
| Product names | Pack Image | PIP code | Date |
| Previous: GLYPRESSIN® injection 1mg powder and solvent for solution for injection vials |  |
03467719 |
End: April 2024 |
| New:
Terlipressin acetate 1mg powder and solvent for solution for injection vials |
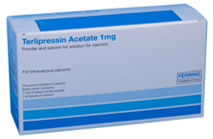 |
127-0974 | Start: May 2024 |
| Previous: GLYPRESSIN® 0.12mg/ml solution for injection ampoules |  |
00178400 | End: April 2024 |
| New:
Terlipressin acetate 0.12mg/ml solution for injection ampoules |
 |
426-2812 | Start: May 2024 |
We want to assure you that this change will not impact the quality or efficacy of our terlipressin acetate products. There will be no alterations to the package size, and any modifications to the packages’ look and feel will be minimal.
If you require any further information, please do not hesitate to contact us.
Commerical-related questions should be directed to:
Ferring Customer Services
0800 1114125
Medical related questions should be directed to:
Ferring Medical
0800 1114126
