

High and sustained patient satisfaction1
With FIRMAGON® administration.1
What do patients think about FIRMAGON®3,4?
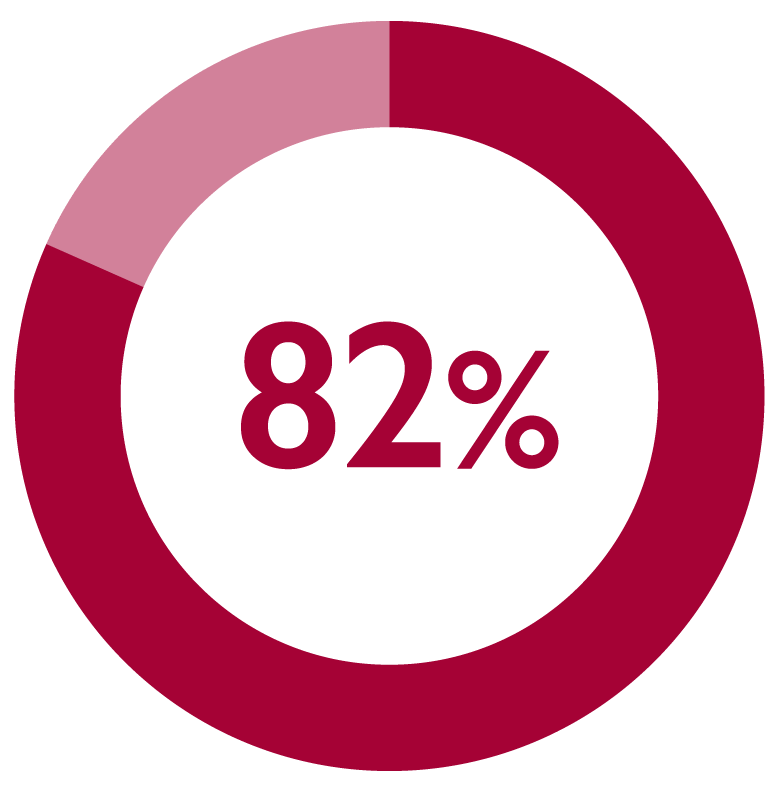
Monthly FIRMAGON® administration was considered satisfactory by 82% of patients (173/211) at 6 months1*
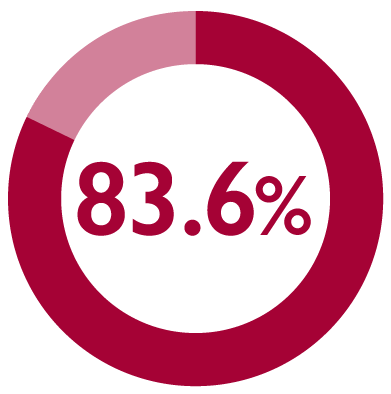
Satisfaction was sustained during the maintenance phase, with patient satisfaction rate increased to 83.6% (117/140) at 12 months1
*Patient satisfaction was the secondary endpoint in a multicentre, long-term, prospective, observational, non-interventional study of FIRMAGON® patients in the Netherlands with advanced prostate cancer. The primary endpoint was PFS failure rate.
What are your key criteria for achieving a positive administration experience for patients?

perceived seeing their urologist every 3 months as ‘just right’1

perceived monthly nurse visits for maintenance injections as ‘just right’1
*Patient satisfaction was the secondary endpoint in a multicentre, long-term, prospective, observational, non-interventional study of FIRMAGON® patients in the Netherlands with advanced prostate cancer. The primary endpoint was PFS failure rate.
Secondary endpoints included patient and physician satisfaction scores.
Optimise your injection technique
Reconstitution and Administration Video Including Top Tips by Leanne McCourt
Summary of Top Tips from Leanne
References
1. Roshani H, et al. Curr Urol 2021;15:204–208.
2. Allchorne P, et al. Int J Urol Nurs 2022;16:127–137.
3. FIRMAGON® 120mg injection Summary of Product Characteristics. Ferring Pharmaceuticals Ltd. October 2022. Available at: https://www.medicines.org.uk/emc/product/6537. Last accessed: January 2024.
4. FIRMAGON® 80mg injection Summary of Product Characteristics. Ferring Pharmaceuticals Ltd. October 2022. Available at: https://www.medicines.org.uk/emc/product/6535. Last accessed: January 2024.
5. Klotz L, et al. BJU Int 2008;102:1531-1538.


