

Discover an ADT in a different class3

FIRMAGON® is a different class of ADT1
LHRH agonists that overstimulate gonadotrophin releasing hormone (GnRH) receptors and rely on a negative feedback to achieve testosterone suppression, but may cause an initial clinical flare.
FIRMAGON®, a GnRH antagonist that blocks GnRH receptors directly, enabling rapid testosterone suppression.
FIRMAGON® is significantly more effective in controlling disease-related symptoms vs. LHRH agonists8
Urinary-tract problems
Urinary-associated problems, e.g. obstructive uropathy leading to persistent infections and LUTS

Bone and back pain
As a result of arthralgia, or eventually bone metastases, which can lead to fractures and spinal cord compression

What do patients think about FIRMAGON®?
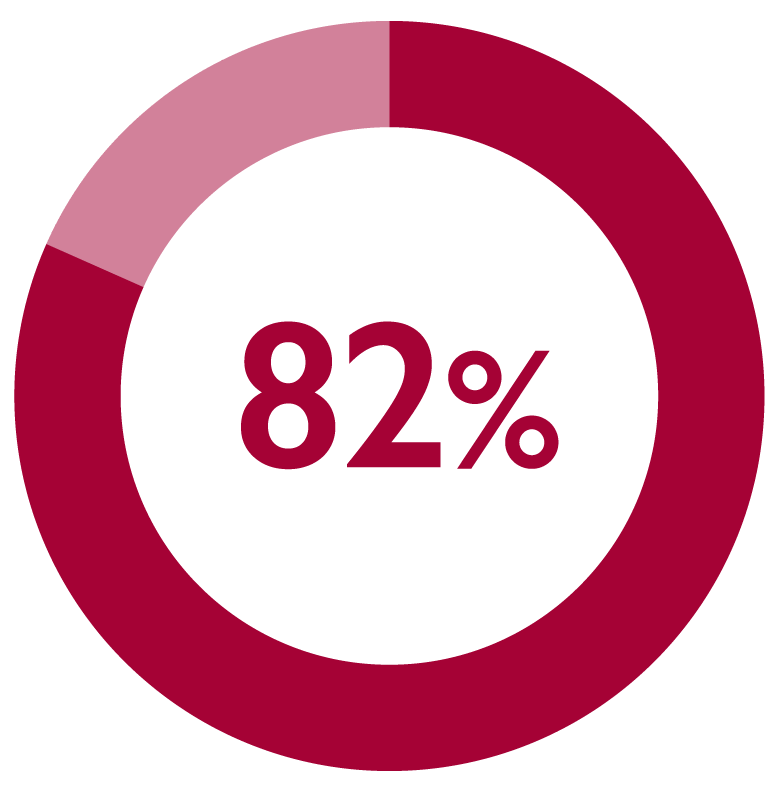
Monthly FIRMAGON® administration was considered satisfactory by 82% of patients (173/211) at 6 months11*
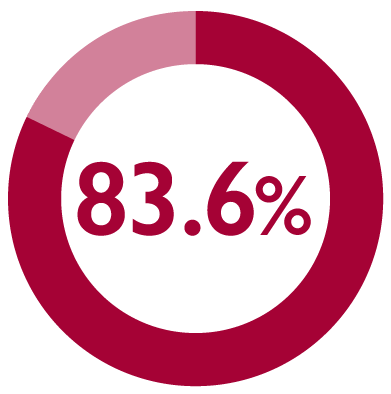
Satisfaction was sustained during the maintenance phase, with patient satisfaction rate increased to 83.6% (117/140) at 12 months11
*Patient satisfaction was the secondary endpoint in a multicentre, long-term, prospective, observational, non-interventional study of FIRMAGON® patients in the Netherlands with advanced prostate cancer. The primary endpoint was PFS failure rate.
FIRMAGON® extended licence
Adapted from European Association of Urology Guidelines, 202215
References
1. Drudge-Coates L. Int J Urol Nurs 2009;3(3):85–92.
2. Plummer C, et al. Trends Urol Men’s Health 2017;13–18.
3. Chowdhury S, et al. BJU Int 2013;112(2):182-9.
4. Gandaglia G, et al. Clin Genitourin Cancer 2015;13:e123–e130.
5. Albertsen et al. Eur Urol 2014 Mar;65(3):565-73
6. Hospital Episode Statistics (HES) database. (Data for Jan-Dec 2021).
7. Data on file, Ferring Pharmaceuticals Ltd. Based on HES data, Xiang-Ming et al., 2017, NICE 2011.
8. Klotz L, et al. Eur Urol 2014;66:1101–1108.
9. Crawford ED, et al. Urology 2014;83:1122–1128.
10. Boccon-Gibod L, et al. Ther Adv Urol 2011;3:127–140.
11. Roshani H, et al. Curr Urol 2021;15:204–208.
12. FIRMAGON® 120 mg injection Summary of Product Characteristics. Ferring Pharmaceuticals Ltd. October 2022. Available at: https://www.medicines.org.uk/emc/product/6537. Last accessed: June 2024.
13. FIRMAGON® 80 mg injection Summary of Product Characteristics. Ferring Pharmaceuticals Ltd. October 2022. Available at: https://www.medicines.org.uk/emc/product/6535. Last accessed: June 2024.
14. Relugolix Summary of Product Characteristics. Available at: https://www.accord-healthcare-products.co.uk/products/o/orgovyx-tablets. Last accessed: June 2024.
15. EAU – EANM – ESTRO – ESUR – ISUP – SIOG Guidelines on Prostate Cancer, 2022. Available at: https://uroweb.org/guidelines/prostate-cancer. Last accessed: June 2024.






