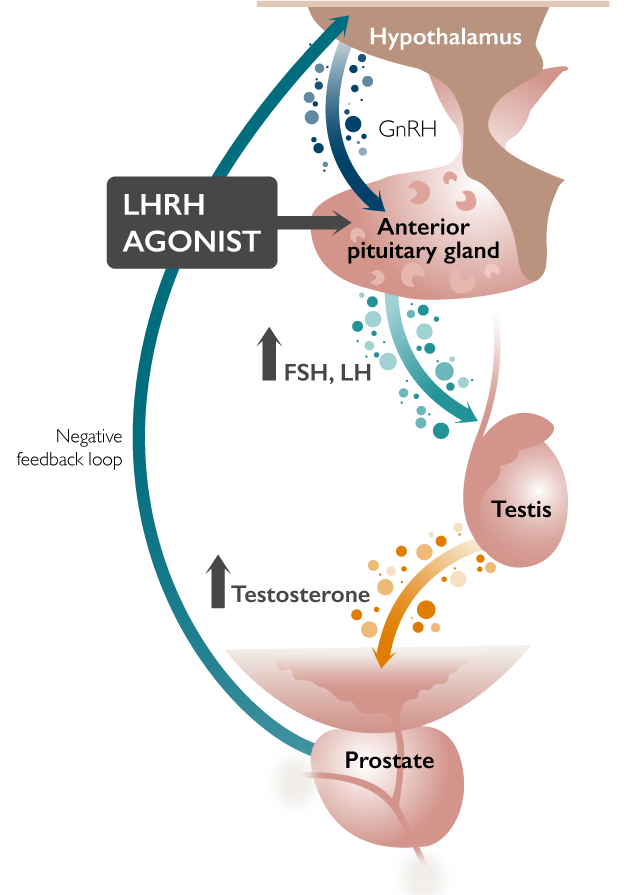


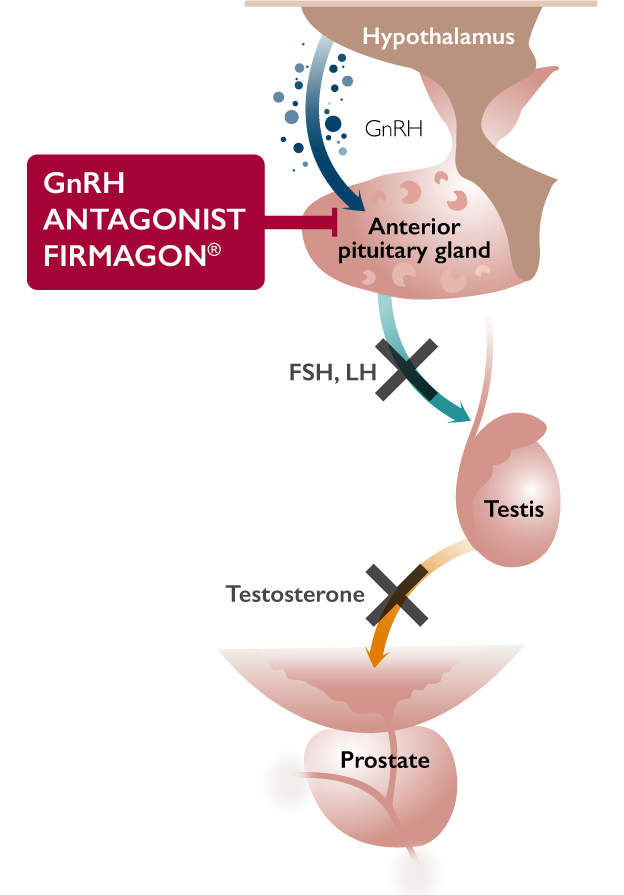
FIRMAGON® is an ADT in a Different Class1
FIRMAGON® is a GnRH antagonist, but how does this differ from an agonist?

FIRMAGON® is associated with fewer systemic adverse events vs. LHRH agonists4,5



Adapted from Klotz L, et al. 20143
Consult the SmPCs for full safety information2,3
*p<0.05. Treatment-emergent AEs occurring over 1 year with FIRMAGON® and LHRH agonists (>5% in any treatment group; pooled analysis of five prospective, randomised Phase III trials).
†LHRH agonists include goserelin and leuprorelin.
Watch How FIRMAGON®s MOA Works
References
1. Drudge-Coates L. Int J Urol Nurs 2009;3(3):85–92.
2. FIRMAGON® 120mg injection Summary of Product Characteristics. Ferring Pharmaceuticals Ltd. October 2022. Available at: https://www.medicines.org.uk/emc/product/6537. Last accessed: January 2024.
3. FIRMAGON® 80 mg injection Summary of Product Characteristics. Ferring Pharmaceuticals Ltd. October 2022. Available at: https://www.medicines.org.uk/emc/product/6535. Last accessed: January 2024.
4. Klotz L, et al. Eur Urol 2014;66:1101–1108.
5. Klotz L, et al. BJU Int 2008;102:1531-1538.



