

FIRMAGON® can delay disease progression1,2,5
Time is incredibly valuable to you and your patients. See how FIRMAGON® can give you more.
FIRMAGON® increases PSA progression-free survival at 12 months vs. LHRH agonists1,5
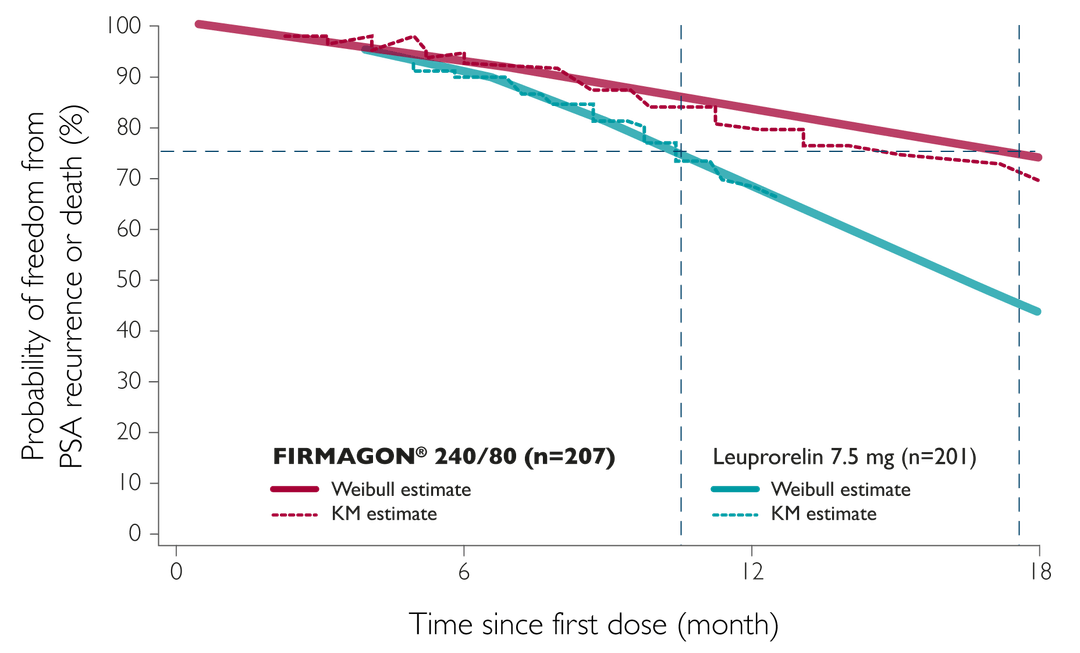
- Delays PSA failure or death by 7 months in high-risk patients** and can maintain this response long-term2
- Increases PSA progression-free survival at 12 months,1 with response maintained to 5 years in significantly more high-risk patients vs. LHRH agonists2
Adapted from Boccon-Gibod L, et al. 2012
*In a long-term extension of a Phase 3 trial. Delay defined as time taken for 25% of patients considered to be at high-risk (with a baseline PSA ≥ 20 ng/ml) to experience PSA failure or death (TTP 25%).
TTP25% was significantly longer with FIRMAGON® than leuprorelin (514 vs. 303 days, p=0.001).
**As calculated using the Weibull estimate.
† The primary endpoint of the trial was suppression of testosterone to ≤ 0.5 ng/ml at all monthly measurements from day 28 to day 364
FIRMAGON® provides effective treatment for high-risk prostate cancer patients1-3
*High risk defined as patients with baseline PSA ≥20 ng/ml.1,2
References
1. Crawford ED, et al. Urology 2014;83:1122–1128.
2. Boccon-Gibod L, et al. Ther Adv Urol 2011;3:127–140.
3. Chang AJ, et al. Nat Rev Clin Oncol 2014;11(6):308–323.
4. Klotz L, et al. Eur Urol 2014;66:1101–1108.
5. Tombal B, et al. Eur Urol 2010;57:836–842.


