

REKOVELLE® (follitropin delta) is indicated for controlled ovarian stimulation for the development of multiple follicles in women undergoing assisted reproductive technologies (ART) such as in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) cycle1
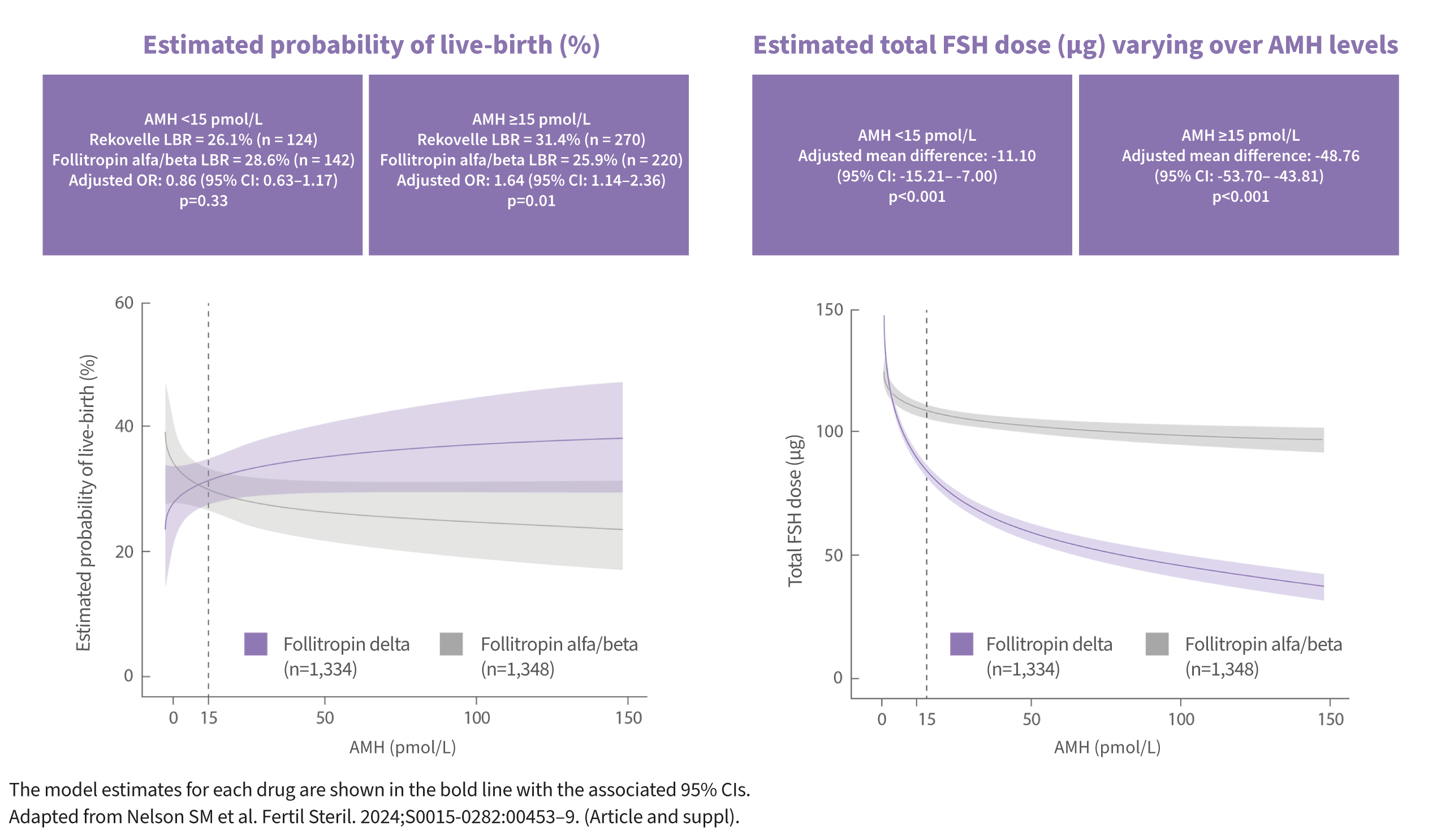
REKOVELLE® has demonstrated a 64% increased probability of live-birth in patients with AMH≥15 pmol/L, with a lower dose of gonadotrophin compared with follitropin alfa/beta in an individual participant data meta-analysis of randomised controlled trials7*
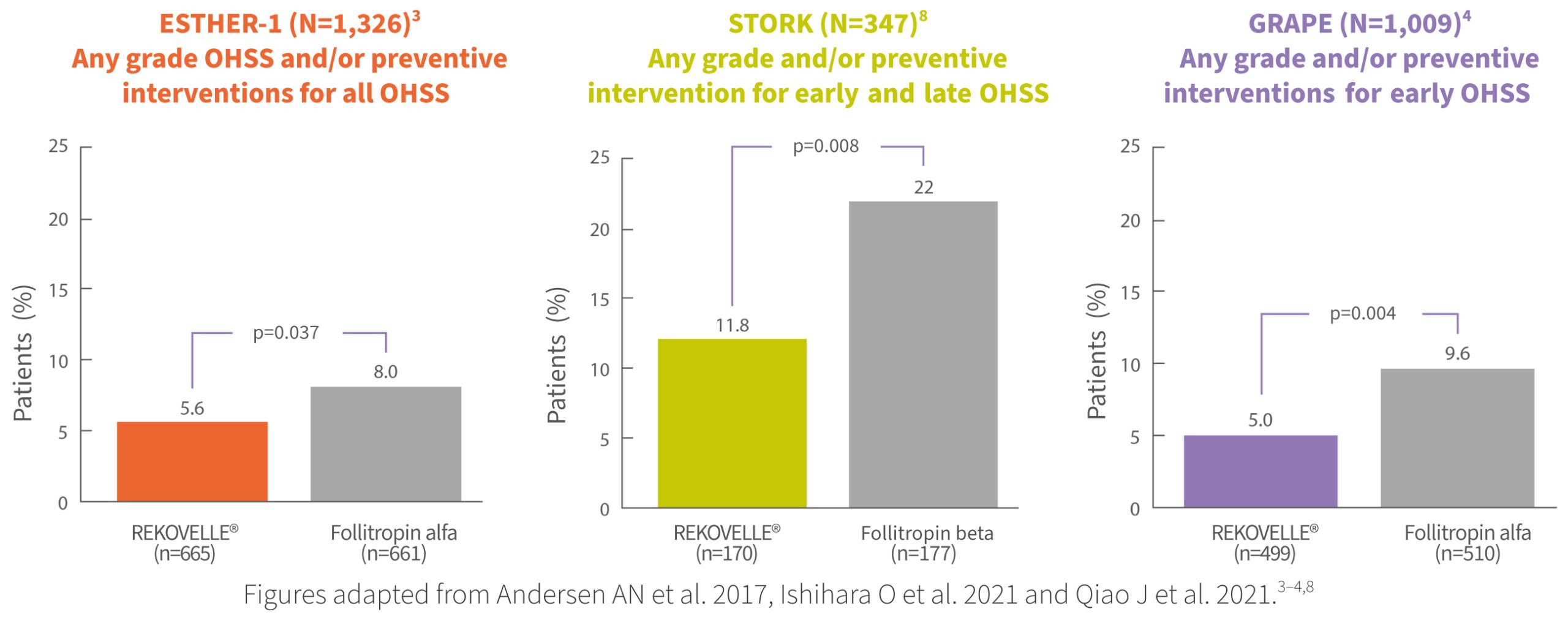
REKOVELLE® has consistently demonstrated significantly lower rates of OHSS and/or preventive interventions, compared with follitropin alfa/beta, across three Phase 3 clinical trials3-4,8†
REKOVELLE® is the only human-cell-line-derived rFSH and, in a Phase 1 study, had a higher biopotency compared with follitropin alfa1,2
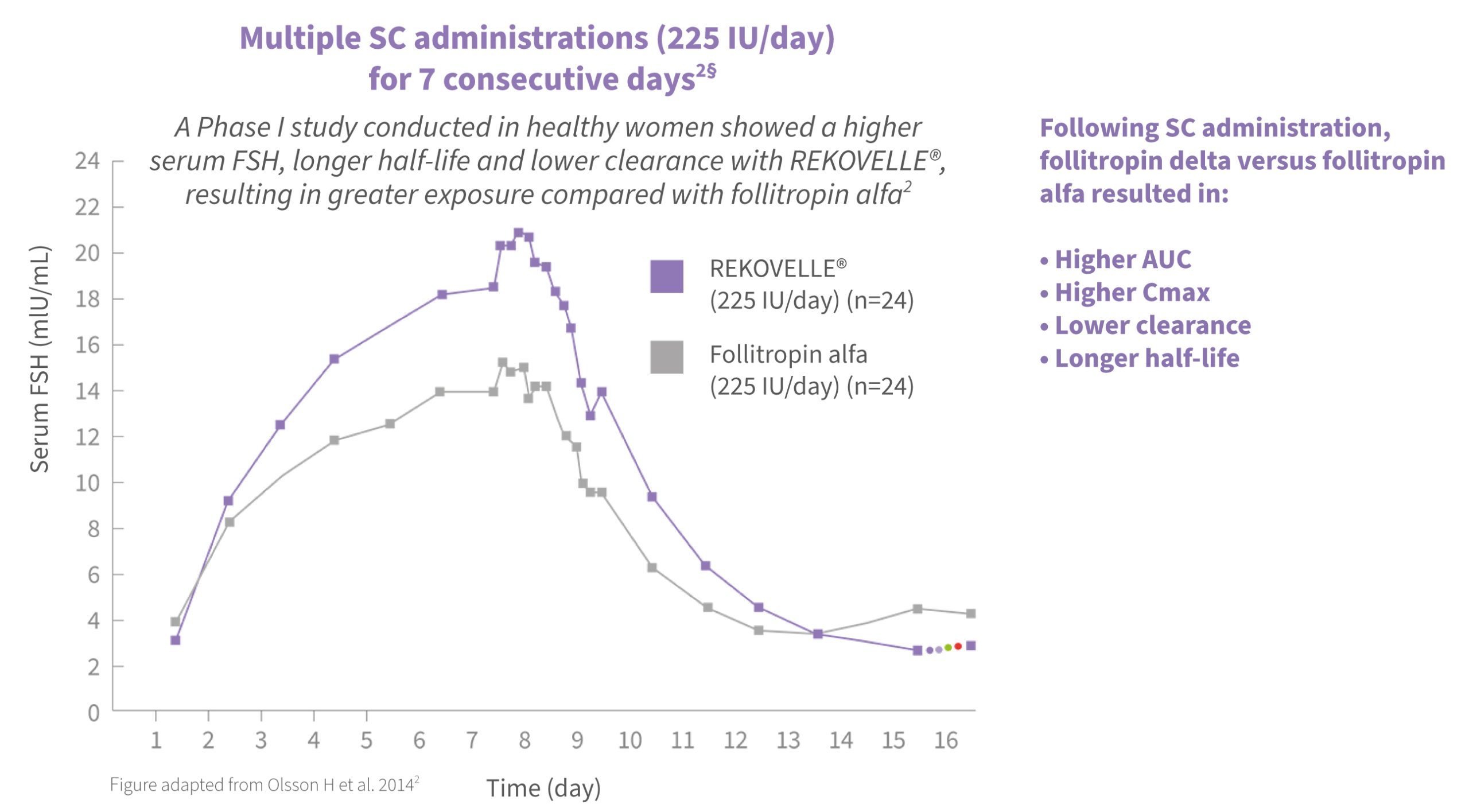
It is not clear to what extent the different properties of these rFSH products translates into clinical practice.2
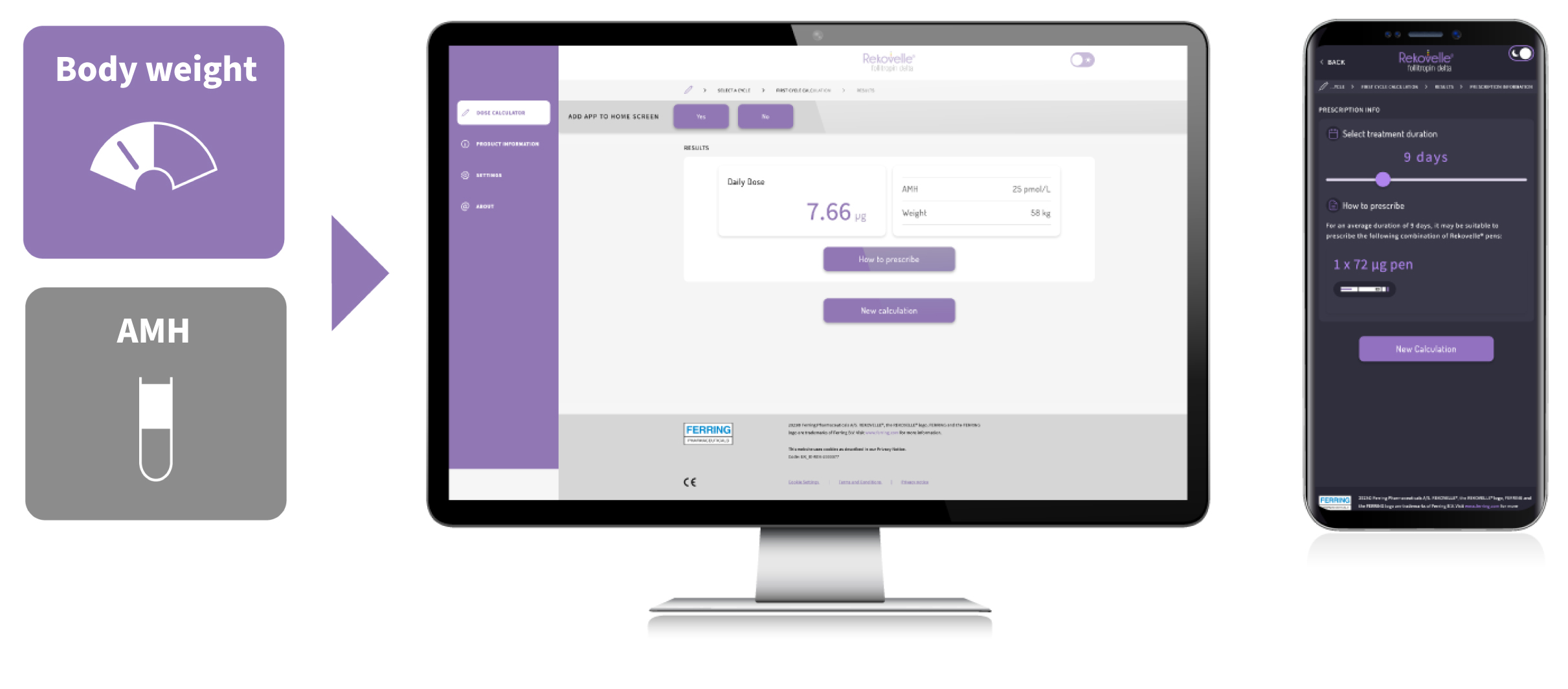
REKOVELLE® algorithm provides an individualised, fixed dose (μg), based on body weight and AMH, with no need for dose adjustments in the cycle9-11
REKOVELLE® pre-filled pen has the shortest and thinnest needle vs other pens for gonadotrophin administration12

References
1. REKOVELLE® (follitropin delta) solution for injection in a pre-filled pen. Summary of Product Characteristics.
Available at: https://www.medicines.org.uk/emc/search?q=rekovelle or https://www.medicines.ie/medicines/rekovelle-34826/spc;
2. Olsson H et al. J Clin Pharmacol. 2014;54:1299–1307;
3. Andersen AN et al. Fertil Steril. 2017;107:387–396;
4. Ishihara O et al. Reprod Biomed Online 2021;42:909–918;
5. Howles CM. Hum Reprod Update 1996;2:172–191;
6. Latash WD et al. Rev Gynaecol Pract 2004;4:203–210;
7. Nelson SM et al. Fertil Steril. 2024;S0015-0282:00453–9. (Article and suppl);
8. Qiao J et al. Hum Reprod. 2021;36:2452–2462;
9. Arce JC et al. In Anti-Müllerian Hormone (Nova Science Publishers, Inc., 2016);
10. Rose TH et al. Drugs R D 2016;16:173–180;
11. REKOVELLE® (follitropin delta) dosing calculator. Available at: https://www.dosedelta.ferring.com/calculator (Last accessed May 2025);
12. Ferring Pharmaceuticals. Data on file – REF-15085;
13. mylife Clickfine DiamondTip. Available at: https://www.mylife-diabetescare.com/en-GB/products/pen-needles/mylife-clickfine-diamondtip.html (Last accessed May 2025).
Footnotes
* Individual participant data meta-analysis of randomised controlled trials. Primary efficacy endpoint: Live birth per cycle started. When looking at all patients, there was no significant difference in live birth rates between REKOVELLE® compared with follitropin alfa/beta.
† REKOVELLE® met the non-inferiority primary endpoint (ongoing pregnancy rate) in the GRAPE study4 and in the STORK study (number of oocytes retrieved).8 REKOVELLE® also met the non-inferiority co-primary endpoints (ongoing pregnancy rate and ongoing implantation rate) in the ESTHER-1 study.3 OHSS and/or preventive interventions for OHSS were safety outcomes in the ESTHER-1, STORK and GRAPE studies. No adjustment for multiplicity was made on secondary endpoints and, therefore, it is not possible to draw any conclusions from these findings.3-4,8 OHSS is a common AE with an incidence of (≥1/100 to 1/10).4
§ REKOVELLE® should be dosed in μg as per the algorithm in the Summary of Product Characteristics.1
Abbreviations
CHO, Chinese hamster ovary; IU, international units; (r)FSH, (recombinant) follicle-stimulating hormone; SC, subcutaneous; AMH, anti-Müllerian Hormone; CI, confidence interval; FSH, follicle-stimulating hormone; OR, odds ratio;
AE, adverse event; OHSS, ovarian hyperstimulation syndrome; RWE, real-world evidence.
