A PERSONALISED APPROACH TO IVF




Adverse Event reporting information can be found in footer
Request a Meeting
How to work out your patient’s Rekovelle® dose in the first treatment cycle.
Rekovelle® first treatment cycle
ACCESS THE REKOVELLE® DOSING CALCULATOR
Access Code: Fgb2
STEP 1
Round your patient’s anti-müllerian hormone (AMH) value* to the nearest integer
*AMH measured within the previous 12 months using the ELECSYS® AMH Plus immunoassay from Roche, ACCESS® AMH Advanced from Beckman Coulter or LUMIPULSE G® AMH from Fujirebio.
Using this rounded AMH value, obtain the appropriate dosage factor for your patient from the table below
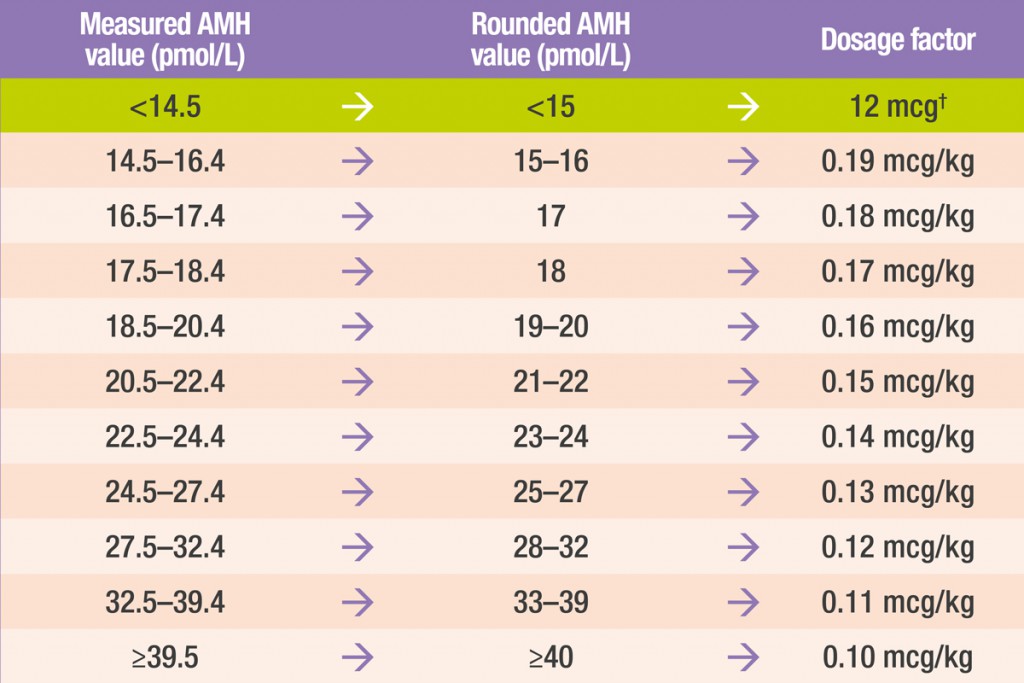
AMH conversion: 1 ng/mL = 7.14 pmol/L
†For women with an AMH level of <15 pmol/L, the daily dose of Rekovelle® for the first treatment cycle is 12 μg, irrespective of body weight.
Measure your patient’s body weight (kg) and multiply by the appropriate dosage factor based on their AMH value
| Body weight (kg) | x | Dosage factor | = | Daily REKOVELLE® dose |
|---|
Round the calculated dose to the next 0.33 μg
EXAMPLES
| Example 1 | |
|---|---|
| AMH Value | 13 pmol/L |
| Body Weight | 59 kg |
| Daily dose of Rekovelle® | |
| Fixed daily dose: 12 μg | |
| Example 2 | ||
|---|---|---|
| AMH Value | 15.5 pmol/L-> Factor = 0.19 μg/kg | |
| Body Weight | 60 kg | |
| Daily dose of Rekovelle® | ||
| 60 kg x 0.19 μg/kg | = 11.4 μg | Round dose to the nearest 0.33 μg -> 11.33 μg |
| Example 3 | ||
|---|---|---|
| AMH Value | 21.5 pmol/L-> Factor = 0.15 μg/kg | |
| Body Weight | 65 kg | |
| Daily dose of Rekovelle® | ||
| 65 kg x 0.15 μg/kg | = 9.75 μg | Round dose to the nearest 0.33 μg -> 9.66 μg |
Note: The maximum daily dose for the first treatment cycle is 12 μg.
Once determined, the daily dose of Rekovelle® is maintained without further adjustment during the treatment cycle.
| Response in the previous cycle | Adjustment of the daily dose in the previous cycle | ||
|---|---|---|---|
| A | Adequate ovarian response without development of OHSS | → | Same daily dose |
| B | Low ovarian response | → | Increase daily dose by 25% or 50% (based on the extent of the response) |
| C | Ovarian hyper-response | → | Decrease daily dose by 20% or 33% (based on the extent of the response) |
| D | Occurrence of OHSS or risk of developing OHSS in previous cycle | → | Decrease dose so it is 33% lower than the dose used in the cycle where OHSS or risk of OHSS occurred |
OHSS, ovarian hyperstimulation syndrome.
Note: The maximum daily dose for subsequent treatment cycles is 24 μg. Once determined, the daily dose of Rekovelle® is maintained without further adjustment during the treatment cycle.
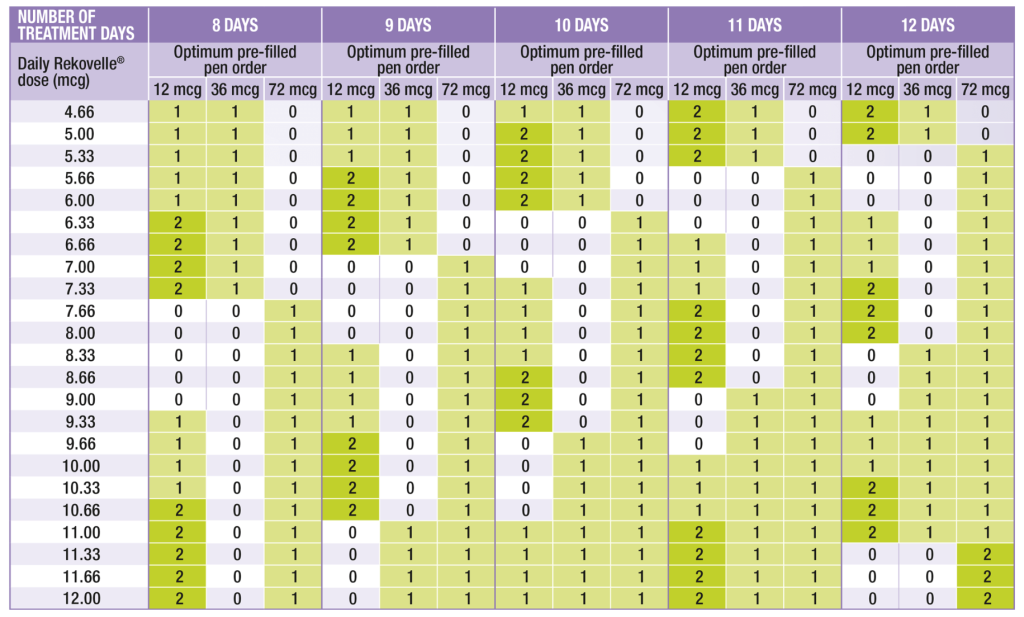
The table below can be used to calculate the optimal number and sizes of Rekovelle® cartridges to prescribe according to the determined daily dose and expected number of treatment days, to help minimise wastage.
Job Code: UK-REK-2300031 - Date of preparation: November 2023