CLINICAL IMPROVEMENT AND REMISSION RATES

Adverse Event reporting information can be found in footer
Request a Meeting
CLINICAL IMPROVEMENT AND REMISSION RATES
COHORT INFORMATION
DISTRIBUTION AND EXTENT OF UC
TIME TO SYMPTOM RESOLUTION1
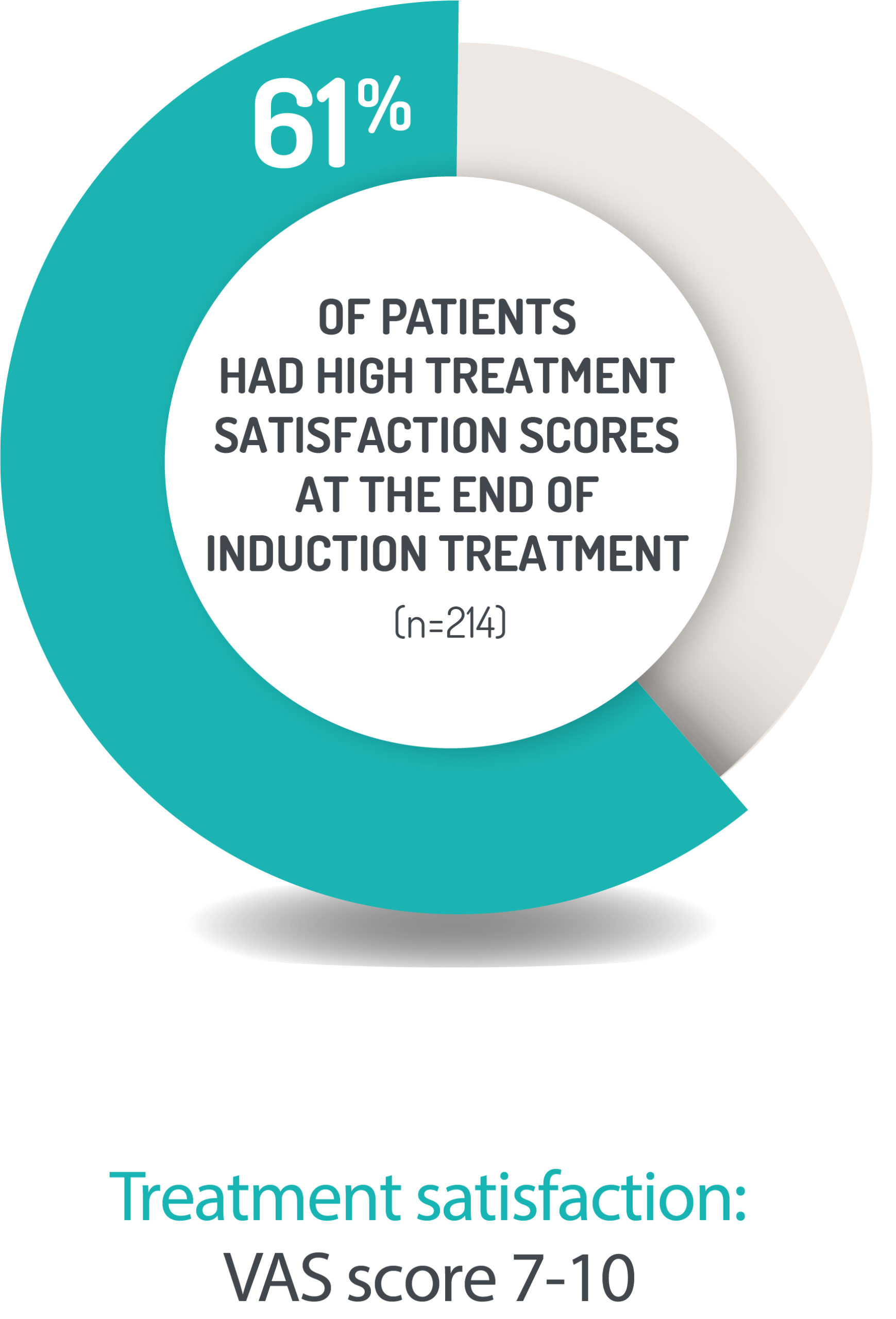
PATIENTS ARE HIGHLY SATISFIED WITH CORTIMENT TREATMENT1
FURTHER RESULTS


![]() 1 x 9mg CORTIMENT® tablet OD for up to 8 weeks2
1 x 9mg CORTIMENT® tablet OD for up to 8 weeks2
| Patient Numbers | Dose Schedule |
|---|---|
| 59 (16.9%) | CORTIMENT® added >14 days after 5-ASA optimisation for current flare |
| 260 (74.5%) | CORTIMENT® added ≤14 days after 5-ASA optimisation for current flare or without dose modification |
| 30 (8.6%) | CORTIMENT® monotherapy |
![]() 1 x 9mg CORTIMENT® tablet OD for up to 8 weeks2
1 x 9mg CORTIMENT® tablet OD for up to 8 weeks2

![]() 1 x 9mg CORTIMENT® tablet OD for up to 8 weeks2
1 x 9mg CORTIMENT® tablet OD for up to 8 weeks2
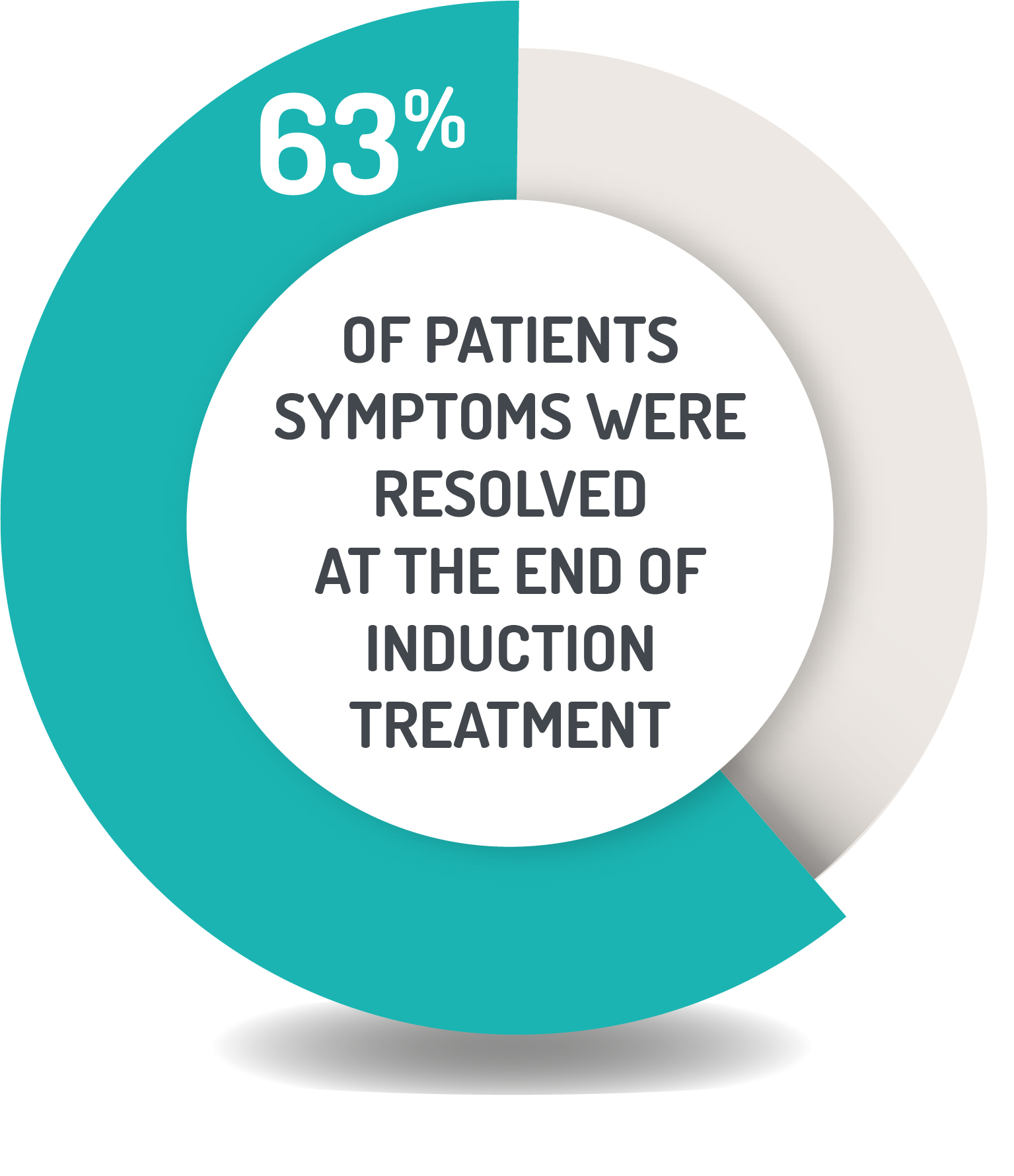

Symptom resolution: Rectal bleeding score of 0 and stool frequency score of 1
![]() 1 x 9mg CORTIMENT® tablet OD for up to 8 weeks2
1 x 9mg CORTIMENT® tablet OD for up to 8 weeks2

![]() 1 x 9mg CORTIMENT® tablet OD for up to 8 weeks2
1 x 9mg CORTIMENT® tablet OD for up to 8 weeks2

![]() 1 x 9mg CORTIMENT® tablet OD for up to 8 weeks2
1 x 9mg CORTIMENT® tablet OD for up to 8 weeks2
STUDY SUMMARY
Results from a prospective, multi-centre, observational cohort study assessing
the effectiveness of CORTIMENT for mild to moderate active UC
| Patient Numbers | Dose Schedule |
|---|---|
| 59 (16.9%) | CORTIMENT® added >14 days after 5-ASA optimisation for current flare |
| 260 (74.5%) | CORTIMENT® added ≤14 days after 5-ASA optimisation for current flare or without dose modification |
| 30 (8.6%) | CORTIMENT® monotherapy |
BASELINE CHARACTERISTICS 1
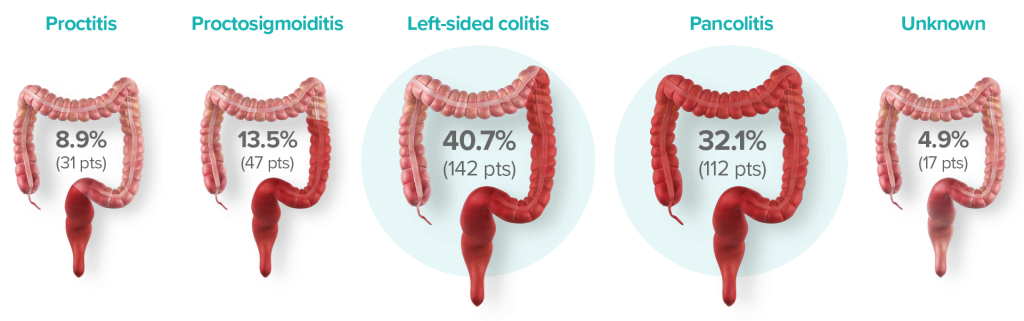
*Intention-to-Treat (IIT) population
SAFETY RESULTS1
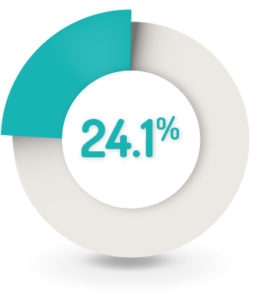
24.1% (n=84) of patients reported at least one AE

14.3% (n=50) of patients discontinued CORTIMENT due to AEs
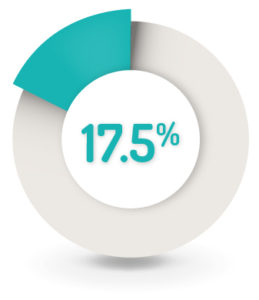
17.5% (n=61) of patients reported at least one AE related to the study drug
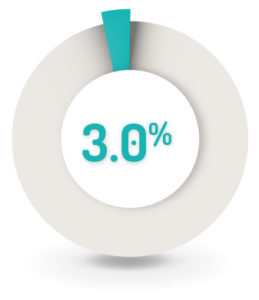
3% (n=9) of patients had worsening of the underlying UC
Job Code: UK-COR-2200001 - Date of preparation: January 2022