MENOPUR® is indicated in women undergoing controlled ovarian hyperstimulation to induce multiple follicular development in patients undergoing assisted reproductive technologies; and for anovulation, including polycystic ovarian disease in women who have been unresponsive to treatment with clomiphene citrate1
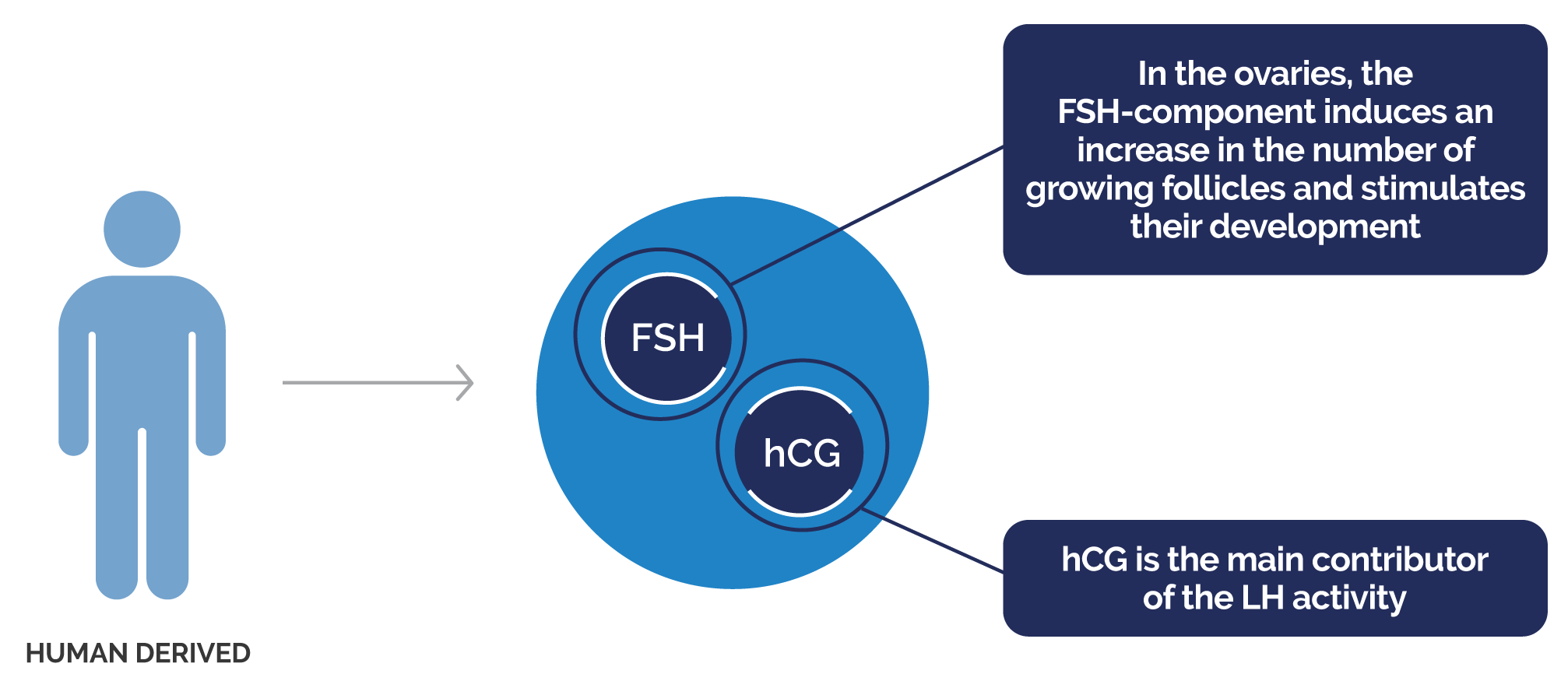
- MENOPUR® is a urinary derived gonadotrophin that has both LH and FSH activity in a 1:1 ratio1
- MENOPUR® has been used to treat over 4.4 million patients globally over the last 20 years2
MENOPUR®’s hCG-DRIVEN LH ACTIVITY PROMOTES A FAVOURABLE ENDOCRINE ENVIRONMENT THAT HAS BEEN SHOWN TO PRODUCE A HIGHER PROPORTION OF TOP-QUALITY EMBRYOS COMPARED TO rFSH ALFA3*
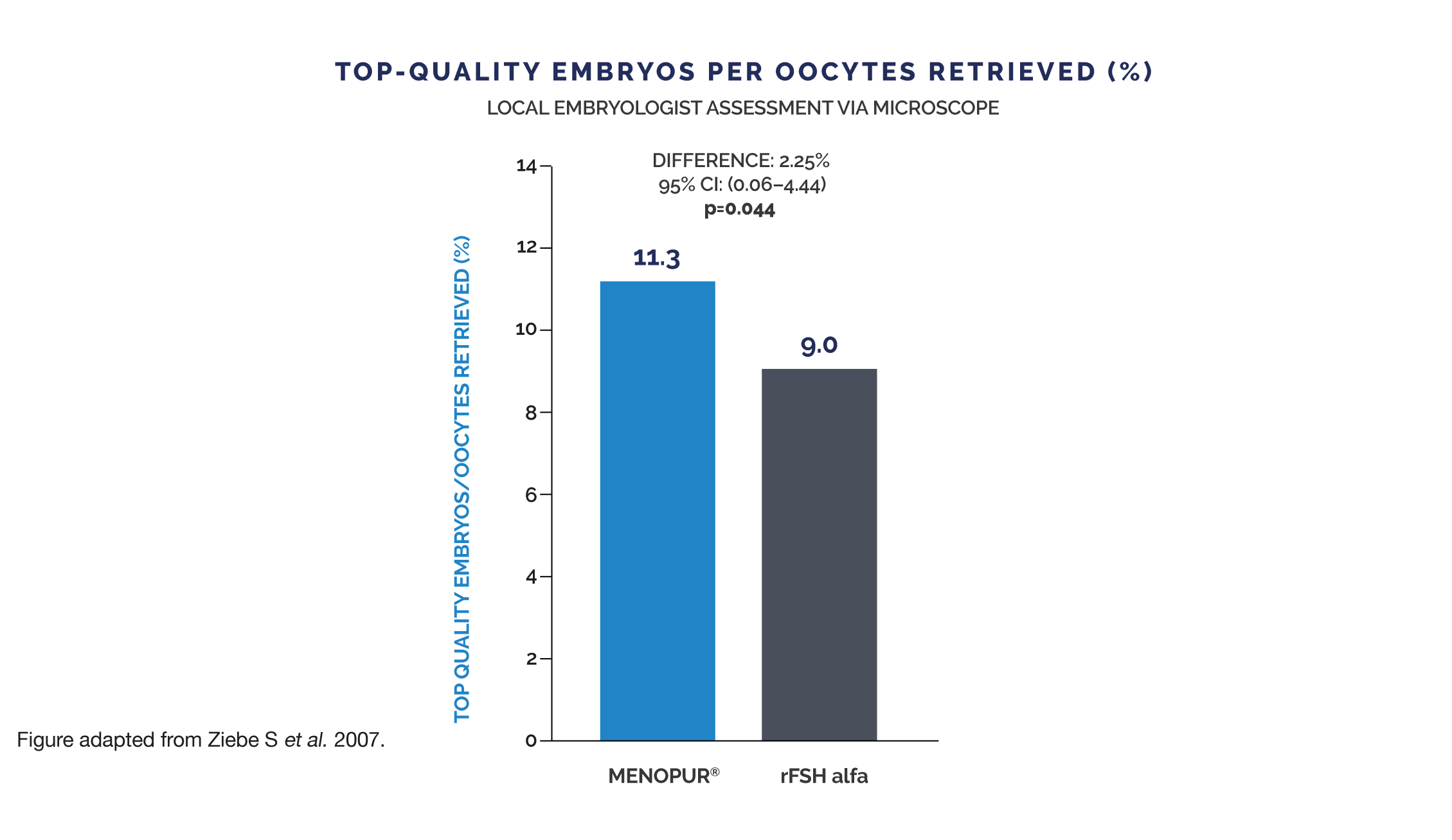
Embryo quality was also assessed by a panel of central embryologists using representative pictures of each embryo. Observations by the central embryologists were in the same direction (MENOPUR® 9.5% vs rFSH alfa 8.0%), but significance was not reached.
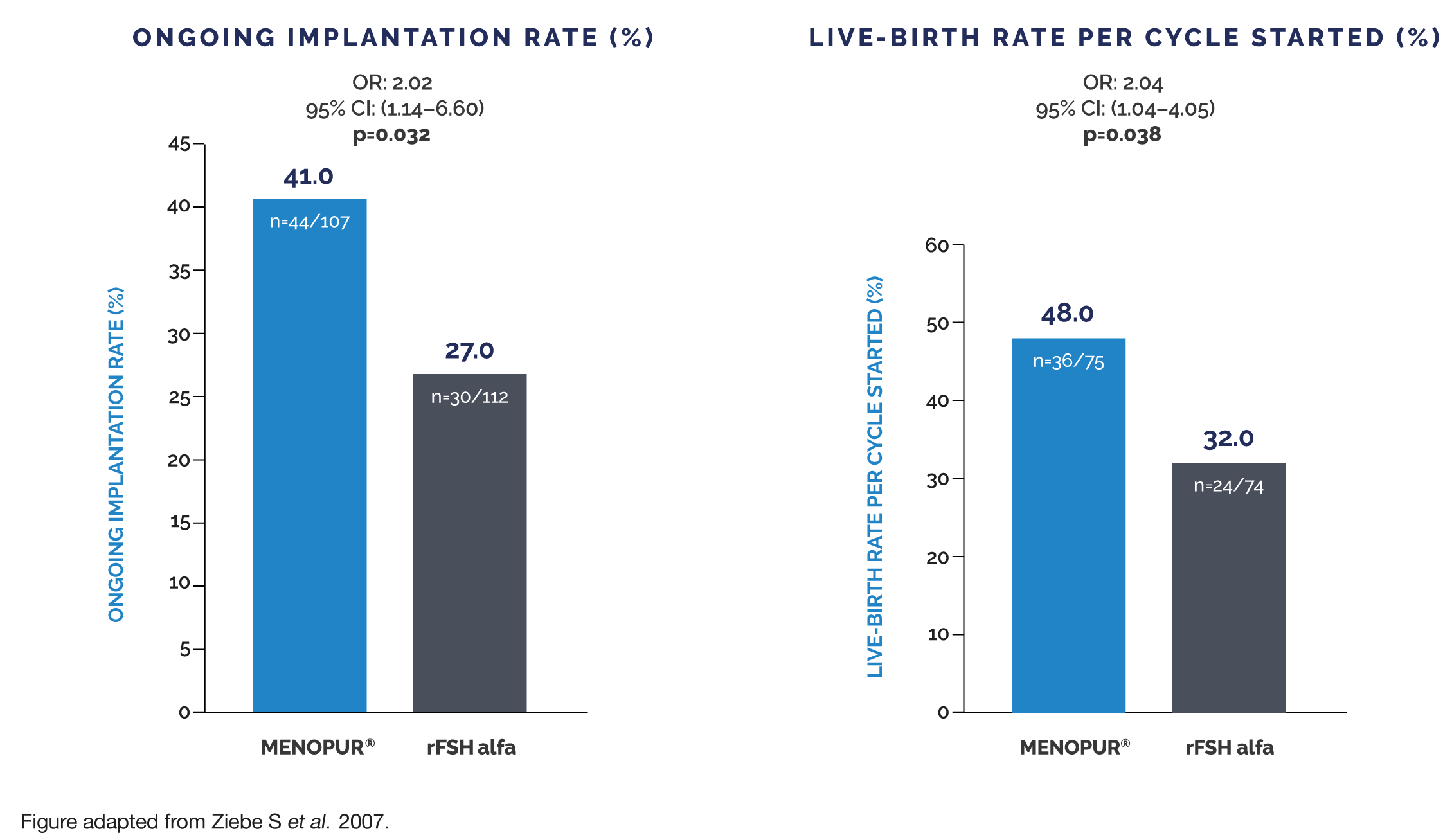
MENOPUR® HAS SHOWN SIGNIFICANTLY INCREASED IMPLANTATION AND LIVE-BIRTH RATES COMPARED TO rFSH ALFA WHEN ONLY TOP-QUALITY EMBRYOS TRANSFERRED3†
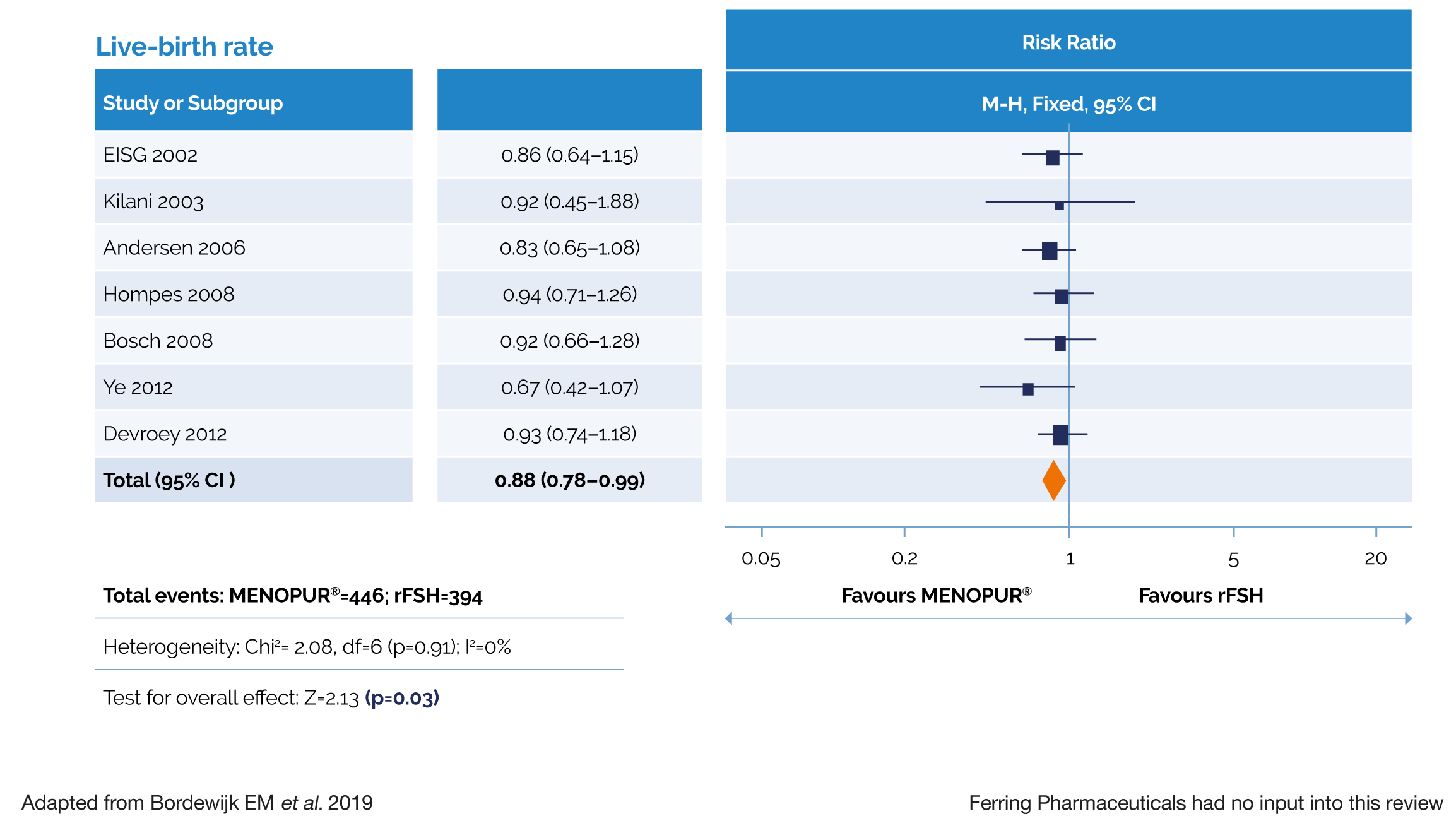
MENOPUR® DEMONSTRATED A SIGNIFICANTLY HIGHER LIVE-BIRTH RATE COMPARED WITH FOLLITROPIN ALFA/BETA IN A LARGE SYSTEMATIC REVIEW AND META-ANALYSIS4‡
MENOPUR® OFFERS A RANGE OF FORMULATIONS TO MEET YOUR PATIENTS’ NEEDS
MENOPUR® multidose 600 IU and 1200 IU:
• Convenient
• Easy to use
• Solvent provided as a pre-filled syringe1
• Can be stored at room temperature for a maximum of 28 days once reconstituted1
PROVEN PATIENT SATISFACTION WITH MENOPUR® MULTIDOSE§
The MENOPUR® multidose patient satisfaction survey in the United Kingdom found (N=96):5

References
1. MENOPUR® (menotrophin) Summary of Product Characteristics. Available at: https://www.medicines.org.uk/emc/search?q=menopur;
2. Ferring. DOF: REF-22783;
3. Ziebe S et al. Hum Reprod. 2007;22:2404–2413;
4. Bordewijk EM et al. Hum Reprod Open. 2019:hoz008;
5. Shaker et al. 2018. MENOPUR® Multidose Patient Satisfaction Questionnaire Survey. Poster presented at British Fertility Society Meeting. Liverpool 2018.
Footnotes
* This evaluation was a retrospective analysis of the MERiT trial. MENOPUR® met the non-inferiority primary endpoint (ongoing pregnancy rate) in the MERiT trial. Proportion of top-quality embryos per oocyte retrieved was a secondary endpoint in the MERiT trial. No adjustment for multiplicity was made on secondary endpoints and, therefore, it is not possible to draw any conclusions from these findings. Top-quality embryos were defined as 4–5 blastomeres on Day 2,
≥7 blastomeres on Day 3, equally-sized blastomeres and ≤20% fragmentation on Day 3 and no multinucleation.
† This evaluation was a retrospective analysis of the MERiT trial. MENOPUR® met the non-inferiority primary endpoint (ongoing pregnancy rate) in the MERiT trial. Live-birth rate and ongoing implantation rates were secondary endpoints in the MERiT trial. No adjustment for multiplicity was made on secondary
endpoints and, therefore, it is not possible to draw any conclusions from these findings. Top-quality embryos were defined as 4–5 blastomeres on Day 2
≥7 blastomeres on Day 3, equally-sized blastomeres and ≤20% fragmentation on Day 3 and no multinucleation.
‡ The primary endpoint of the meta-analysis was the total amount of gonadotrophins used (IU) per woman who started an IVF/ICSI cycle per live birth. When pooling data from all studies, there was no evidence of a difference in the amount of gonadotrophins required with MENOPUR® vs rFSH.
Live birth rate was a secondary endpoint in this meta-analysis. No adjustment for multiplicity was made on secondary endpoints and, therefore, it is not possible to draw any conclusions from these findings.
§ A questionnaire survey was conducted across three UK CARE fertility clinics.
Abbreviations
FSH, follicle-stimulating hormone; hCG, human chorionic gonadotropin; LH, luteinising hormone; CI, confidence interval;
MERiT, Menotrophin versus Recombinant FSH in vitro Fertilisation Trial; (r)FSH, (recombinant) follicle-stimulating hormone; OR, odds ratio; df, degrees of freedom; ICSI, intracytoplasmic sperm injection; IU, international units; IVF, in vitro fertilisation; M-H, Mantel-Haenszel.