PREVENTING PREMATURE LUTEINISING HORMONE (LH) SURGE 1



Adverse Event reporting information can be found in footer
Request a Meeting
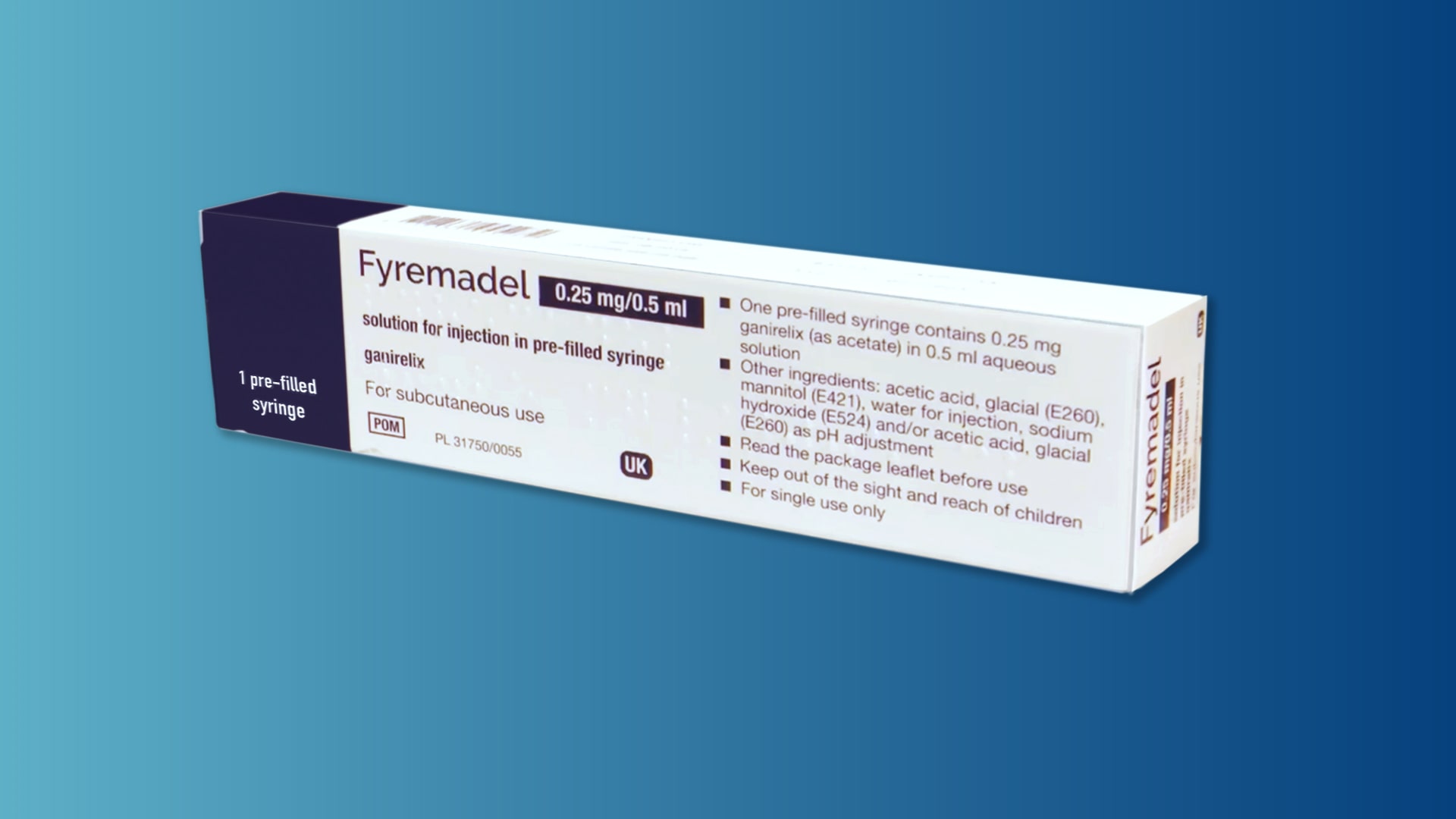
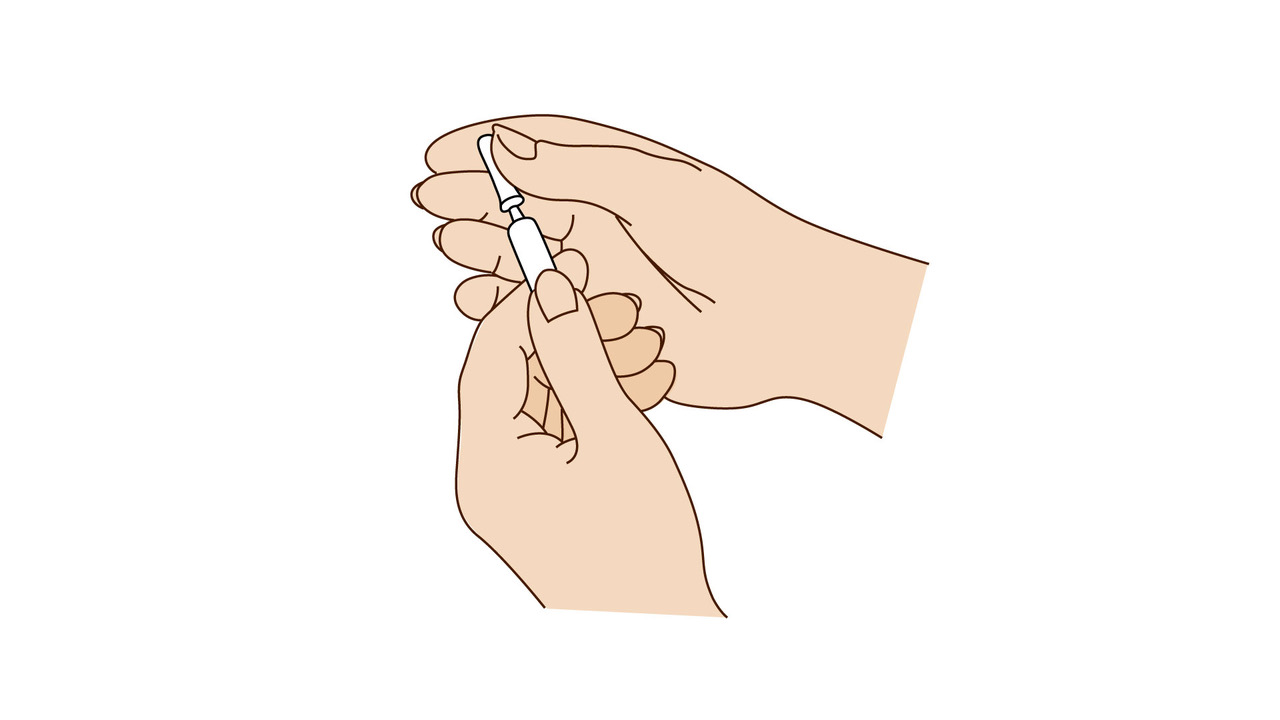
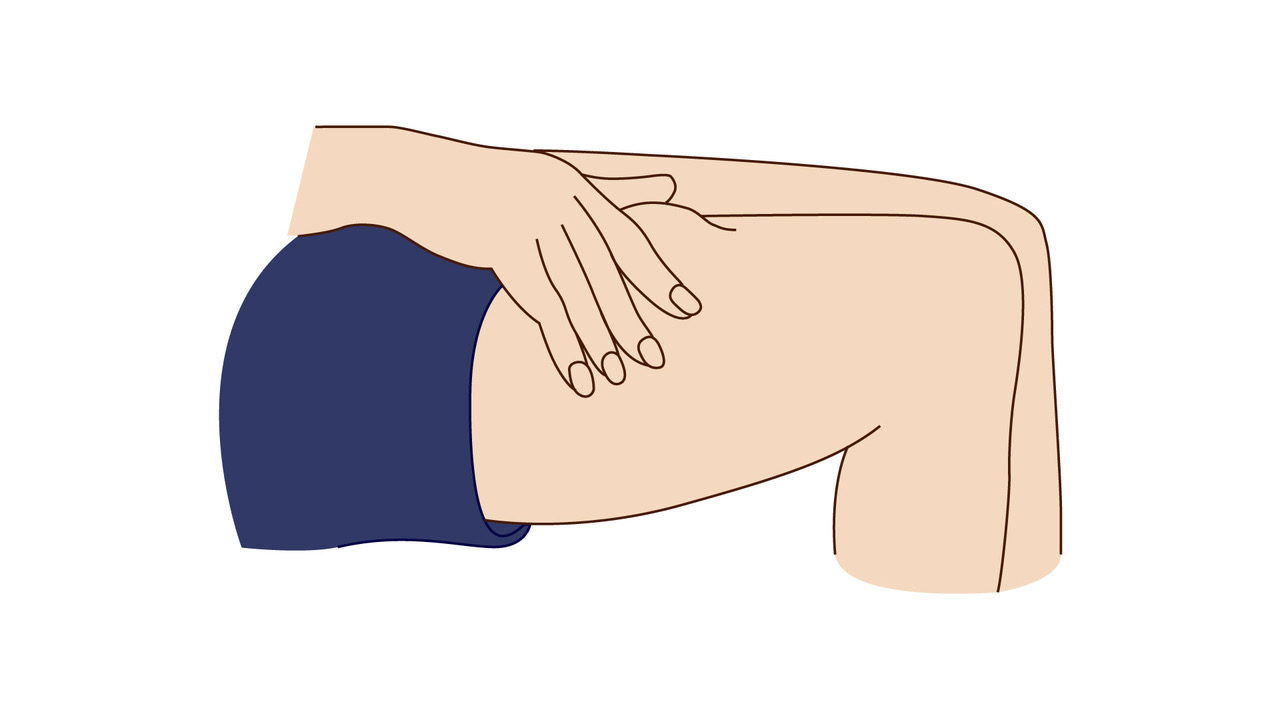
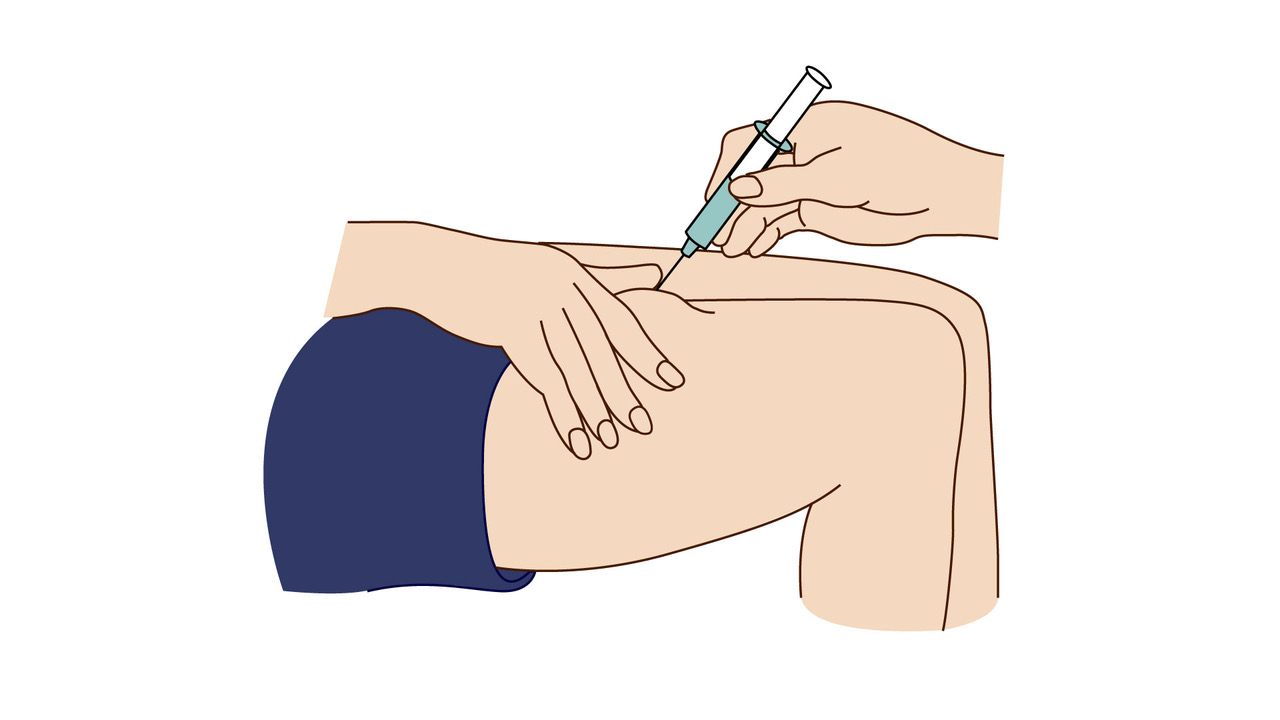
A step-by-step guide which you may find helpful when demonstrating how to use Fyremadel® with your patients.
Fyremadel® is supplied in pre-filled syringes that contain one daily dose. You should only use a Fyremadel® pre-filled syringe once, after which it should be disposed of by placing into a sharps container.

Report any side effects to your fertility clinic or pharmacist. This includes any possible side effects not listed in the package leaflets. Patients can also report side effects directly via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard.
Adverse events should also be reported to Ferring Pharmaceuticals Ltd.
Tel: 0800 111 4126 Email: medical.uk@ferring.com
Fyremadel is a Sun Pharma product and adverse events should also be reported to them. Sun Pharma medical information email: medinfoeurope@sunpharma.com or call their Medical Information Direct Line 0208 848 5052.
By reporting side effects patients can help provide more information on the safety of these medicines.
Job Code: UK-FYR-2300004 - Date of preparation: September 2023